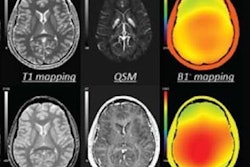
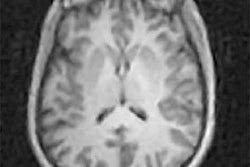
SpinTech has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Spin-SWI software.
The software is designed to combine susceptibility-weighted imaging (SWI) data from MRI scans with SpinTech's proprietary postprocessing techniques to provide enhanced visualization of neurovascular biomarkers in small vessels in the brain.
Spin technology has been used to process more than 7,000 research cases in the study of conditions such as traumatic brain injury, vascular dementia, stroke, and Parkinson's disease, according to the company.