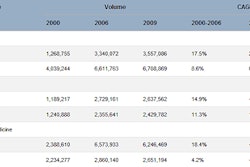
The U.S. Food and Drug Administration (FDA) has published a guidance document for medical device vendors that spells out how it considers the benefits and risks of certain medical devices during premarket review.
The guidance outlines the systematic approach that FDA device reviewers take when making benefit-risk determinations during the premarket review process, according to the agency. It also provides manufacturers with a tool that explains the various principal factors considered by the agency during the review of premarket approval (PMA) applications; the regulatory pathway for high-risk medical devices; and de novo petitions, a regulatory pathway for novel, low-to-moderate-risk devices.
In addition, the guidance describes an approach that takes into account patients' tolerance for risks and perspectives on benefits, as well as the novelty of the device, the FDA said. The guidance can be found here.