CHICAGO -- PET/CT scans can identify patients on lutetium-177 prostate-specific membrane antigen-617 (Lu-177 PSMA-617) therapy who are at risk of potentially life-threatening adverse events, according to research presented December 1 at the RSNA meeting.
The finding is from an analysis of 237 patients with metastatic castration-resistant prostate cancer (mCRPC) who underwent treatment between June 2022 and January 2025, with a significant number experiencing severe hematologic adverse events (AEs), such as anemia and thrombocytopenia.
“[Lu-177 PSMA-617] is a life-prolonging treatment in mCRPC and is used in later lines, when patients often have bone involvement and limited marrow reserve,” said Alireza Ghodsi, MD, a fellow at the University of Washington in Seattle, who received a RSNA trainee research prize for the work.
Lu-177 PSMA-617 (Pluvicto, Novartis) was approved by the U.S. Food and Drug Administration (FDA) in March 2022 for adults with prostate-specific membrane antigen (PSMA)-positive metastatic cancer who have not responded to standard treatments. Hematologic adverse events are common in patients, with results from the two main trials of the drug suggesting that grade 3 anemia may occur in up to 13% of patients and grade 3 thrombocytopenia in 11%.
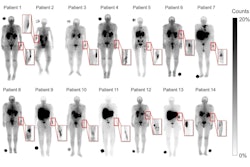
PSMA-PET/CT allows clinicians to measure the extent of bone involvement based on PSMA radiotracer uptake (bone tumor volume), and in this study, the researcher hypothesized that the imaging metric could allow them to predict hematologic AEs.
The study involved 237 patients (median age, 73 years old) undergoing treatment at the Fred Hutchinson Cancer Researcher Center and the University of Iowa who received a median of three cycles of Lu-177 PSMA-617. Ninety-one percent of patients had received prior chemotherapy (59% with one line, 32% with ≥2 lines), while 64% had prior radiation and 13% received radium-223 treatment.
Hematological parameters were recorded at baseline and every three weeks during treatment. AE severity was assessed using standard criteria, with baseline PSMA-PET/CT scans quantitatively analyzed with a semiautomated whole-body tumor segmentation software.
According to the results, 56 patients (17%) experienced grade ≥3 anemia, 14 (4%) grade ≥3 leukopenia, and 23 (7%) grade ≥3 thrombocytopenia. An analysis revealed that patients with higher PSMA-PET/CT bone volume at baseline developed the AEs in significantly shorter time (p < 0.001). Moreover, the association remained significant after adjusting for other baseline patient characteristics, Ghodsi noted.
“Higher PSMA-PET bone tumor volume was significantly associated with increased risk of grade 3 and 4 anemia and thrombocytopenia, and it remained significant when we adjusted for other risk factors,” Ghodsi said.
Ultimately, identifying patients at higher risk for AEs during treatment can inform attempts for early recognition and help provide better supportive care to improve patient outcomes, he concluded.
Visit our RADCast for full coverage of RSNA 2025.