Thyrotoxicosis
Thyrotoxicosis refers to the syndrome caused by excess free thyroid hormone (thyroxine) [8]. Hyperthyroidism denotes increased metabolic activity of the thyroid gland- therefore, a hyperthyroid patient is thyrotoxic, but a thyrotoxic patient need not have an overactive thyroid [8]. In other words, thyrotoxicosis can be the result of excess hormone production by the thyroid gland (Graves' Disease- in which case the RAIU will be increased) or it can occur secondary to other conditions including: Exogenous administration of thyroid hormone (in which case the RAIU and thyroglobulin will be decreased), thyroiditis (RAIU generally decreased), struma ovarii (ectopic hormone production), or choriocarcinoma (production of TSH-like substance which stimulates the thyroid gland).
Diffuse Toxic Goiter (Graves Disease):
Pathophysiology
Graves disease is the most common cause of thyrotoxicosis in a population with adequate iodine intake (about 150 ug/d in adults) [7,10]. Graves' disease is an autoimmune disorder characterized by the presence of a TSH receptor antibody. This antibody was previously referred to as Long-Acting Thyroid Stimulator (LATS) but is currently more appropriately called as Thyroid Stimulating Immunoglobulin (TSI) or Thyroid-Stimulating Antibody (TSAb). TSI is an IgG antibody that reacts with TSH receptors on follicular cells. The antibody stimulates the cell as though the receptor was being triggered by TSH, thus inducing constant excessive function, hyperplasia, and hypertrophy of the gland. TSI is found in 90 to 95% of cases of Graves disease.
Clinical Findings
The age of onset is most commonly between 30-40 years. Females are much more commonly affected than men (7:1). Patients present with a goiter, exophthalmos, tachycardia, weight loss, heat intolerance, hyperactive reflexes, warm skin, lid lag, and/or a tremor. Patients older than 75 years are more likely to present with subtle symptoms or atrial fibrillation [10]. The thyroid gland may be normal sized in a small number of patients.
Extrathyroidal manifestations include an infiltrative
ophthalmopathy which may cause
pain, excessive tearing, blurred vision, and diplopia. Other
findings include lid
retraction, periorbital edema, optic neuritis, and enlargement of
the extraoccular
muscles. Another extrathyroidal manifestation is an infiltrative
dermopathy (pretibial
myxedema). There is no increased risk of thyroid cancer in these
patients (spontaneous
incidence of thyroid cancer in patients with Graves disease is
between 0.5 and 2%).
Thyroid storm is an acute, life-threatening hypermetabolic state that occurs rarely in 1-2% of hyperthyroid patients [9]. If untreated, the mortality rate can be as high as 20% [9]. Symptoms of thyroid storm include two or more of the following: fever (>100? F), severe tachycardia, high pulse pressure, agitation with tremors, delirium, flushing, sweating, heart failure, nausea, vomiting, diarrhea, and jaundice [9]. About 50% of patients that present with thyroid storm report a dramatic weight loss [9]. Thyroid storm can be triggered by infection (particularly pulmonary infection), vigorous palpation of the thyroid gland in hyperthyroid patients, diabetic ketoacidosis, hyperosmolar coma, insulin-induced hypoglycemia, attempted surgical treatment for hyperthyroidism, withdrawl of anti-thyroid medication, and I-131 therapy (very rare) [9]. Treatment consists of supportive care with antipyretics, antithyroid medication, and beta-blockers [9].
T3 and T4 levels are elevated and TSH is decreased in patients with Graves disease. T3 levels are typically three times normal, while T4 values are usually double. Early in the disease, only T3 toxicosis may be seen, with the FT4I falling within normal limits. There is a flat TSH response to TRH stimulation in patients with Graves disease.
Neonates born to mothers with Graves disease are at an increased risk for hyperthyroidism secondary to transplacental passage of thyroid stimulating antibodies [8]. Onset of symptoms is within hours to days and death can result in 15% of cases. With proper therapy the condition will resolve spontaneously over a period of about 3 months as the thyroid stimulating antibody disappears.
Imaging
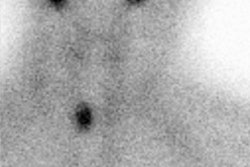
Scintigraphy with either I-123 or Tc04 is useful in excluding other causes of thyrotoxicosis. The scan generally demonstrates a uniform distribution of increased activity in an enlarged gland, with decreased background activity, and an elevated RAIU. If iodide turnover is rapid, early RAIU values will be markedly elevated, but may be normal by 24 hours. The pyramidal lobe, a variant projection of thyroid tissue arising from the isthmus or the medial aspect of one of the lobes and ascending superomedially, is more frequently visualized by scintigraphy in the presence of Graves' disease (up to 40% of patients). In about 10% of cases, the thyroid is normal in size [2].
Ultrasound evaluation of the thyroid in Graves' disease demonstrates markedly increased vascular flow and velocity suggests thyroid hyperfunction.
|
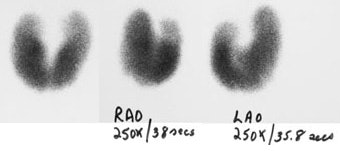
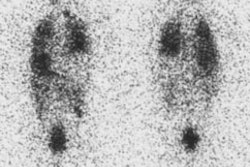
Graves Disease: Pinhole images from a Tc-99m pertechnetate thyroid exam demonstrate diffuse thyroid enlargement with decreased background activity. Scan times for 250,000 count images are markedly decreased in this patient with Graves disease. |
|
|
|
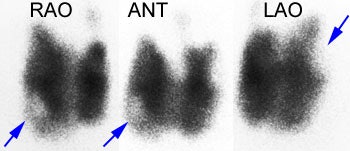
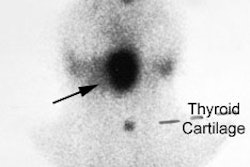
Graves disease and papillary carcinoma: The Tc-99m images below are from a patient with Graves disease. Cold nodules (blue arrows) can be seen in the lower pole of the right lobe and the upper pole of the left lobe. Although these could represent TSH dependent nodules, the lesions in this case were related to multifocal papillary thyroid carcinoma. Although patients with Graves disease are not at an increased risk for thyroid cancer, the lesion may be more aggressive in this patient population [J Clin Endoc Metab 1990; Belfiore A et al. Increased aggressiveness of thyroid cancer in patients with Graves' disease. 70:830-835]. |
|
|
Marine-Lenhart Syndrome:
The coexistence of a TSH dependent nodule(s) and Graves' disease is referred to as Marine-Lenhart syndrome [5]. These nodules will appear as cold areas on thyroid scans because of TSH suppression associated with Graves'. Following I-131 therapy, however, the nodules may persist and accumulate radiotracer as the TSH level starts to rise. Biopsy of cold nodules in patients with Grave's disease should still be considered strongly as up to 9% are malignant [6].
|
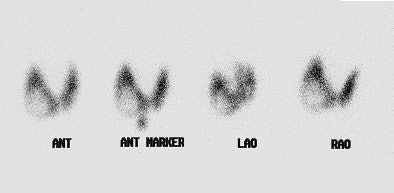
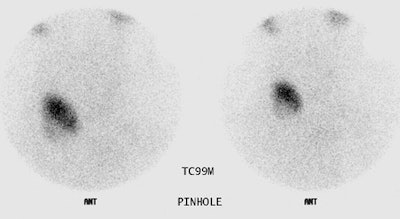
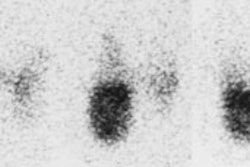
Marine-Lenhart syndrome: The patient shown below presented with hyperthyroidism and a 24 hour I-123 uptake of almost 60%. A large cold nodule was noted on the study (upper row of images). A biopsy of the nodule prior to treatment indicated a benign nodule. Following ablation therapy with I131 the patient remained markedly hyperthyroid. A follow-up technetium scan 2 months after treatment demonstrated tracer concentration within the previously cold nodule (lower images) consistent with Marine-Lenhart syndrome. |
|
|
Treatment for Graves disease
1- Surgery
Sufficient thyroid tissue is removed to reduce overall hormone output. Frequently, too little or too much thyroid is removed. Surgery is not the preferred method of treatment because of the potential complications of general anesthesia and the possibility of recurrent laryngeal nerve damage.
2- Antithyroidal drugs
Antithyroid drugs such as the thionamides- Propylthiouracil (preferred during pregnancy [7]) and Tapazole (Methimazole- has a longer half-life that allows for once a day dosing) act by blocking the intrathyroidal organification (iodination) of the tyrosine residues on the thyroglobulin molecule by interacting with the enzyme thyroid peroxidase- thereby inhibiting thyroid hormone formation. Therapeutic actions of these agents begin as early as 4 to 12 hours after administration.
The agents may also alter thyroid immunoregulatory mechanisms which are central to the etiology of this autoimmune disorder (may serve to decrease circulating antibody titers by having a direct inhibitory effect on antibody synthesis by thyroid lymphocytes). PTU has the added extrathyroidal effect of exerting a non-competitive inhibition on the peripheral conversion of T4 to T3.
The medication is prescribed for 12-18 months [7] and these agents can control symptoms of hyperthyroidism in 90% of patients. Permanent remission may be achieved in 20 to 30% of patients [7]. Unfortunately, relapse rates are frequent (about 50%) even in patients who are treated for over 2 years. Relapse rates are higher (approaching 90%) in patients treated for less than 2 years.
Side effects include fever, rash, pruritis (3-5%), and neutropenia. Rare, but serious side effects include arthritis, vasculitis, hepatitis, and agranulocytosis (which is idiosyncratic and is most likely to occur within the first few weeks to months of therapy). Agranulocytosis is seen in about 0.35% of patients, but is more rare when the dose of methimazole is lower than 30 mg daily [7]. Methimazole can also cause an obstructive liver disorder [7]. In pregnant patients, methimazole may cause aplasia cutis (a scarlike lesion of the scalp) and has also been linked to choanal atresia [7]. The risk of side effects increases with increasing doses of the agents. Agranulocytosis is treated with recombinant human granulocyte colony-stimulating factor (rhG-CSF) and has been reported to be effective in shortening the recovery time of neutrophils in patients with thionamide induced-agranulocytosis [1].
Solitary Toxic Nodule
A solitary toxic nodule is an infrequent cause of hyperthyroidism in the US (but it can account for up to 9% of cases of thyrotoxicosis in Europe) [4]. The risk of developing thyrotoxicosis in an autonomous nodule is higher for older patients and in patients living in areas of relatively low iodine intake [4]. Generally, the nodule must be of a sufficient size (3 cm or larger) to be able to secrete enough hormone to produce thyrotosicosis [7]. Antithyroid drugs are not a treatment option due to the high frequency of recurrence on cessations of the treatment [5]. I-131 is the preferred therapy [5]. Some also refer to a solitary toxic nodule as Plummer's disease [5].
Toxic Nodular Goiter (Plummer's Disease)
In populations with iodine deficiency, toxic multinodular goiter,
a non-autoimmune condition, is the most common cause of
hyperthyroidism [10].
Pathophysiology
Plummer's Disease is characterized by the autonomous function of one or more thyroid adenomas producing hyperthyroidism.
Clinical Findings
The disorder is more commonly seen in patients 40-50 years old. Females are affected more than men (3:1). Patients generally present with mildly elevated thyroid hormone levels, decreased TSH, and a slightly elevated or normal RAIU [5]. Plummer's disease, however, is more frequently associated with T3 toxicosis (i.e.: T3 is elevated in preference to T4) than is Graves' disease.
Imaging
On scintigraphy, there is usually a patchy appearance to the thyroid due to the presence of multiple cold, warm, and hot nodules. One to several autonomous nodules which suppress the activity in the remainder of the gland are usually identified. Suppression, however, does not connote toxicity per se. Autonomously functioning nodules less than 2.5 cm in diameter are rarely toxic and have little likelihood of becoming so unless they enlarge [3]. Nodules larger than 3 cm are much more likely to become toxic (20% by 6 years). Uptake measurements are usually normal or low at 24 hours in a toxic multinodular goiter and generally elevated with a toxic adenoma.
Treatment
Treatment is based upon the patients clinical status. In euthyroid patients (i.e.: hyperfunctioning nodule maintains a normal level of thyroxine) only about 5% of these individuals will progress to become thyrotoxic. The vast majority of patients [80%] with multinodular goiter are euthyroid and have a normal RAIU. Treatment in these patients is observation.
An empirical dose of 25-29 mCi of I-131 is used to treat hyperthyroid patients with multiple nodules. If the patient has a solitary hyperfunctioning nodule, the great majority of the administered dose will be concentrated there, thus protecting the remainder of the thyroid gland and post-therapy hypothyroidism is rarely seen in this setting.
Factitious Hyperthyroidism
Factitious hyperthyroidism occurs when a patient ingests thyroid hormone in sufficient quantities to induce a thyrotoxic state. A helpful diagnostic tool for confirming facticious hyperthyroidism is the thyroglobulin level [5]. Thyroglobulin levels are low or undetectable in facticious hyperthyroidism [8], while other forms of hyperthyroidism will demonstrate normal or elevated thyroglobulin levels. These patients will have decreased RAIU and poor uptake of tracer on thyroid exams.
There is elevation of T3 and T4 which suppress TSH. If T3 alone is ingested, serum T4 is usually depressed as a result of inhibition of thyroid secretion secondary to T3 suppression of TSH. The low T4 values are in contrast to the normal T4 values expected with true T3 toxicosis. The RAIU will be decreased [5].
Iodine induced hyperthyroidism- Jod-Basedow phenomenon:
Iodine induced hyperthyroidism refers to excessive T4 synthesis
and release in an iodine deficient patient upon resumption of
dietary iodine intake or administration of IV contrast. Under
normal conditions, there is a protective mechanism which prevents
increased iodine ingestion from leading to excess thryoid hormone
production [5]. This is known as the Wolff-Chaikoff effect [5].
Lack of this protective effect can occasionally occur-
particularly in older patients with long standing multinodular
goiters living in iodine-deficient areas [5,8]. The disorder
occurs insidiously when there is excessive exposure to iodine-
such as with iodine containing medications (SSKI drops or
amiodarone) [5].
Amiodarone is fat soluable and has a half-life of many months-
deiodination of amiodarone (400 mg daily dose) produces about 12
mg of free iodine daily (normal daily requirement is 150-200ug)
[8]. Other authors suggest a maintenance dose of amiodarone (200
mg/d) contains about 75 mg of iodine [10]. Hyperthyroidism occurs
in 1-10% of amiodarone treated patients [10]. There are two forms-
type 1 occurs in patients as a result of excessive iodine-induced
hormone synthesis and release [10]. Type 2 is a destructive
thyroiditis leading to release of preformed hormone from the
damaged thyroid follicular cells [10]. The two forms can be
distinguished using Tc-sestamibi imaging which shows preserved
tracer uptake in type 1 and reduced or absent uptake in type 2
[10]. Both radioiodine and Tc-pertechnetate will show decreased
uptake in type 1 and type 2 Amiodarone induced thyrotoxicosis
[10].
Antithyroid drugs in high doses are the treatment of choice, with or without the addition of potassium perchlorate, which blocks further iodine uptake by the gland [5].
Thyroid hormone levels are elevated, the TSH level is suppressed, and the RAIU is low (due to excess iodine in the gland) [5].
TSH induced thyrotoxicosis:
TSH hyperthyroidism occurs secondary to a TSH secreting pituitary adenoma- this is an exceedingly rare neoplasm accounting for less than 1% of all pituitary tumors [5,8]. Men and women are affected nearly equally [8]. TSH secreting pituitary adenomas have been associated with MEN I and McCune-Albright syndrome [8]. TSH stimulation leads to increased levels of thyroid hormone and increased RAIU [5]. Unlike other causes of hyperthyroidism, the TSH level will be elevated [5]. The thyroid gland is palpably enlarged and often multinodular because of sustained TSH stimulation [8]. Visual field defects (classically bitemporal hemianopia) can be found in up to 40-50% of patients secondary to compression of the optic chiasm by the pituitary tumor [8].
Gestational Trophoblastic Disease/Choriocarcinoma:
Human chorionic gonadotropin (hCG) is a glycoprotein hormone that shares a common alpha-subunit with TSH [8]. hCG has confirm thyroid-stimulating activity when present in high concentrations in the serum [8]. Patients with hydatidiform molar pregnancies or choriocarcinoma secret large amounts of hCG [8]. Increased thyroid function can occur in 25-64% of cases, but only 5% of cases have clinically significant thyrotoxicosis [8].
Struma ovarii:
Struma ovarii is a teratoma of the ovary that is composed primarily of thyroid epithelium (by definition, more than 50% of it's structure) and will accumulate iodine [8]. Most struma ovarri lesions are benign and fewer than 3% are malignant [8]. Less than 500 cases have been reported in the literature [8]. The tumor is most common in the 5th or 6th decade amd in more common in countries in which goiter in endemic [8]. About 5-20% of strumae produce significant amounts of thyroid hormone [8]. Ascites is evident in one-third of cases [8].
REFERENCES:
(1) J Clin Endoc Metab 1993; Tamai H et al. Treatment of methimazole-induced agranulocytosis using recombinant human granulocyte colony-stimulating factor (rhG-CSF). 77:1356-1360
(2) Thyroid and whole-body imaging. Charkes ND. In The Thyroid, 5th ed. Ed Ingbar and Braverman. Lippincott, Philadelphia, 1986. 458-478
(3) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(4) J Nucl Med 2003; Zingrillo M, et al. Percutaneous ethanol injection plus radioiodine versus radioiodine alone in the treatment of large toxic nodules. 44: 207-210
(5) Radiographics 2003; Intenzo CM, et al. Scintigraphic manifestations of thyrotoxicosis. 23: 857-869
(6) Endocr Pract 2006; American association of clinical endocrinologists and associazione medici endocrinologic medical guidelines for the clinical practice for the diagnosis and managment of thyroid nodules. 12: 63-102
(7) J Nucl Med 2007; Iagaru A, McDougall R. Treatment of thyrotoxicosis. 48: 379-389
(8) J Nucl Med 2008; Mittra ES, et al. Uncommon causes of thyrotoxicosis. 49: 265-278
(9) Ann Nucl Med 2006; Vijayakumar V, et al. Is it safe to treat
hyperthyroid patients with I-131 without fear of thyroid storm?
20: 383-385
(10) J Nucl Med 2021; Mariani G, et al. The role of nuclear
medicine in the clinical management of benign thyroid disorders,
part 1: hyperthyroidism. 62: 304-312