Hyperparathyroidism
Parathyroid Embryology
The parathyroid glands are responsible for calcium homeostasis via the production of parathyroid hormone (PTH) from chief cells [24,29]. PTH raises serum calcium by promoting the renal tubular absoprtion of calcium, decreasing renal tubular absorption of phosphate, and stimulating osteoclastic activity [24]. PTH also stimulated vitamin D production and this raises serum calcium by promoting its absorption by the GI tract [24].
Eighty percent of normal adults have 4 parathyroid glands, but more than 4 may be present in 10-15% and less than 4 glands in 2-5% of cases. The normal glands weigh between 30-50 gm and are only a few millimeters in size (5 to 6 x 3 to 4 x 1 to 2 mm) and are not usually visualized by ultrasound, or scintigraphy. The superior glands arise from the 4th bronchial cleft pouch and have a relatively constant position (over 90% [24]) lateral and posterior to the upper or middle third of the thyroid [29], typically between the inferior and superior thyroid arteries (near the cricothyroid junction [24]). The inferior glands arise from the 3rd bronchial cleft pouch and descend with the thymus. There position is much more variable, but they are usually located at the lower poles of the thyroid gland (69% [24]) within a layer of fat between the thyroid and the longus coli muscle. The most common ectopic location of the inferior parathyroid glands is within the thymic capsule or the superior mediastinum [30]. About 2% of the inferior glands are found in the mediastinum with the thymus [24]. The superior parathyroid glands receive their blood supply from the superior thyroid arteries, their anastamoses, or the inferior thyroid arteries [1]. The inferior parathyroid glands receive their blood supply from the inferior thyroid arteries [1]. Glands located in the mediastinum are frequently supplied by the thymic branch of the internal mammary artery [1].
Primary
Hyperparathyroidism
Patients with primary hyperparathyroidism present with elevated calcium and PTH levels [30]. They also typically have low phosphorous levels because elevated PTH levels reduce the resorption of phophorous by the kidneys [30]. Patients on lithium or thiazide diuretics can present with elevated calcium and PTH levels and these medications should be stopped (if possible) for accurate PTH evaluation [30]. Primary hyperparathyroidism occurs in about 1 in 700 adults [18]. Patients are usually between 40 to 70 years of age and it is more common in women (particularly post menopausal women- the prevalence in women over the age of 50 y is about 2% [37]) who are affected 2 to 4 times more frequently than men [18,29,35]. The majority of affected patients are asymptomatic (75%) [29]. Manifestations of primary hyperparathyroidism include renal calculi, peptitic ulcer disease/gastric ulcers, osteopenia/fractures, hypertension, bone cysts, and mental depression [18,35]. The diagnosis is based upon detection of low serum phosphate levels with elevated serum calcium and parathyroid hormone [18]. Risk factors for parathyroid adenomas include a history of radiation to the neck (including prior I-131 therapy for Graves disease) [21]. The MEN syndromes I and IIA (RET oncogene mutation) are associated with parathyroid hyperplasia [21,37].
In the majority of patients (about 80-90%) primary hyperparathyroidism is the result of a solitary parathyroid adenoma. Occasionally, adenomas can be multiple (3 to 5%). Parathyroid hyperplasia accounts for the remaining 6-15% of cases, with carcinoma only rarely (<1%) associated with hyperparathyroidism and these patients generally have markedly elevated calcium levels. Patients with parathyroid adenomas typically have minimal elevation of their serum calcium levels. Hyperparathyroidism may be familial and is associated with MEN Type I, MEN Type IIA, hyperparathyroidism-jaw tumor syndrome [29], and neurofibromatosis. Affected patients with hereditary disorders often demonstrate multiple gland hyperplasia [29]. Patients who have received XRT to the neck also have an increased incidence of hyperparathyroidism.
Between 80 to 90% of parathyroid adenomas are located near the thyroid gland. Ectopic lesions are noted in about 10-20% of cases and the anterior mediastinum (para-thymic tissues) is the most common ectopic location. About 2-3% of parathyroid adenomas are found within the thyroid gland (typically the lower pole) [21]. Parathyroid adenomas tend to be well encapsulated, benign lesions. They are often very small, although ectopic lesions tend to be somewhat larger.
The main and only curative treatment of primary hyperparathyroidism is surgery [30,37]. Surgery is indicated in patients who present with symptomatic disease such as nephrolithiasis, nephrocalcinosis, renal dysfunction, osteopenia with fracture, osteitis fibrosa cystica, and any altered neurologic function [30]. For asymptomatic patients, surgery is indicated if the serum calcium concenrtration is 1.0 mg/dL above the upper limit of normal, a 24 hour urine calcium is 400 mg or more, there is a reduction in creatinine clearance of more than 30%, the patients has a bone mineral density with a t-score below -2.5 at any site, a vertebral fracture seen on imaging studies, or age younger than 50 years [30,37]. Patients that do not meet the criteria for surgical treatment should be followed carefully with serum calcium levels every 6 months, and annual creatinine and bone density testing [30].
About 95% of patients will be cured with a cervical exploration for parathyroid adenoma without pre-operative imaging studies. As such, imaging of patients presenting with primary hyperparathyroidism was often previously not performed. However, conventional neck exploration is a time-consuming surgical procedure because of the many vulnerable structures in the neck [18]. Minimally invasive targeted parathyroid surgery guided by pre-operative imaging and intraoperative gamma probe holds multiple benefits and is now used at most medical centers [18,21,22,24,30]. The surgical time and hospital stay are reduced, with subsequent decreased costs, and the cosmetic result in also improved [18,21,22]. Also- post-operative fibrotic changes are limited to the area of surgery which facilitates repeat surgery in cases of disease recurrence [18]. The development of unilateral and focused surgical approaches has made it imperative foe imaging to accurately locate parathyroid glands prior to surgery [24]. The surgery can also be performed with locoregional anesthesia in patients with contraindications to general anesthesia [22]. Gamma probe guided surgery is probably best reserved for patients who have a high probability of a solitary parathyroid adenoma on the basis of imaging, significant Tc-sestamibi uptake within the adenoma, and no co-existing Tc-sestamibi avid thyroid nodules (this accounts for about 60-70% of patients with primary hyperparathyroidism) [21,22]. An adenoma is suspected if the radioactivity is more than 20% of the background [30]. The half-life of PTH in the circulation is approximately 2 minutes [30]. PTH has a short plasma half-life (2-4 minutes) [37]. The use of rapid intraoperative PTH testing can predict successful minimally invasive surgery when there is a greater than 50% drop in serum PTH levels to normal or near normal at 5-10 minutes after resection [24,29]. If a drop does not occur, either mutliple parathyroid adenomas or hyperplasia is likely and the patient usually must undergo bilateral neck exploration [29]. In patients with 4-gland hyperplasia, either 3.5 glands are excised, or all 4 glands are excised with some parathyroid tissue reimplanted in the neck (generally in the sternocleidomastoid muscle or in the forearm) [30].
In patients with recurrent or persistent hyperparathyroidism following surgical exploration (between 2-10% will require reoperation due to persistent or recurrent hyperparathyroidism [21,34]), repeat surgery will be curative in only about 60% of patients [15]. The higher failure rate likely relates to a higher likelihood for ectopic parathyroid tissue in this patient population. Pre-operative localization is extremely useful in this setting.
Percutaneous ethanol ablation of parathyroid adenomas has also been described and can be used in patients that are not surgical candidates and in MEN I patients with recurrent hyperparathyroidism (due to increased risk for surgical complications in these patients if re-exploration is required) [31].
Non-scintigraphic Imaging of Parathyroid Adenomas
On ultrasound, adenomas typically appear as homogenenous oval anechoic or hypoechoic discrete masses posterior to the thyroid gland, anterior to the longus coli muscle, and medial to the carotid artery. Color doppler will show a characteristic extrathyroidal feeding vessel (generally a branch off the inferior thyroid artery) entering the gland at one of the poles [24]. Vascularity tends to be is a peripheral distribution [24]. Pre-operative ultrasound has a sensitivity of 34-85%, and a specificity of 77-98% for the detection of parathyroid adenomas [2,3,15,18,21]. However, in a large meta-analysis, sonographic sensitivity for the detection of a solitary adenoma was 79% (95% CI 77-80%), for hyperplasia it was 35% (CI 30-40%), and for double adenomas it was 16% (CI 4-28%) [24]. The sensitivity falls to about 40% in patients who have prior failed surgical explorations [21]. Identification of a prominent feeding vessel with color Doppler can aid in the detection of abnormal glands [3]. A small lymph node can mimic a parathyroid adenoma. Sonography is less sensitive in the detection of parathyroid hyperplasia [3]. The reliability of sonography is very low for ectopic glands located in the mediastinum. In general, a preoperative approach that combines the both anatomic information of US and the physiologic information of scintigraphy has been shown to predict the presence and location of solitary adenomas more accurately than either technique alone [24].
|
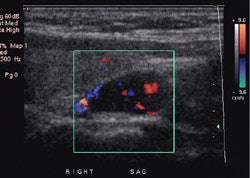
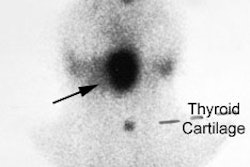
Parathyroid adenoma on ultrasound: A Tc-MIBI exam demonstrated findings consistent with a parathyroid adenoma in the lower right neck. US exam confirmed the presence of a well-circumscribed, vascular hypoechoic nodule below the lower pole of the right lobe of the thryoid. This was a parathyroid adenoma at surgery. |
|
|
CT: On CT, parathyroid adenomas appear as highly vascular
enhancing lesions. The overall sensitivity of CT for the detection
of parathryoid adenomas is between 46
and 87% (which is lower than scintigraphy)
[21,27]. CT sensitivity may be improved
by the use of a dynamic MDCT protocol referred to as 4D CT [33].
The protocol consists of a non-contrast exam, followed by an
arterial phase exam (at 25 seconds following contrast
administration, and a delayed venous phase exam at 80 seconds post
contrast [35]. Classically, parathyroid adenomas demonstrate lower
attenuation than the thyroid on pre-contrast images, early
arterial enhancement greater than the thyroid gland (138-180 HU)
and washout of contrast on delayed phase images, compared to lymph
nodes which demonstrate progressive enhancement over time (lymph
nodes also demonstrate a fatty hilus) [33,35,38,43]. However, this
classic appearance is seen in only 20% of adenomas [43]. Although
thyroid tissue also demonstrates prominent enhancement, it is
usually of higher attenuation on non-contrast imaging [35]. 4D CT
may be superior to sestamibi SPECT/CT imaging (sensitivity 79% vs
58% in one study) [41].
The exam can be degraded by beam hardening associated with the
patients clavicles and shoulders [35]. Cystic change and
intralesional hemorrhage in larger parathyroid adenomas can result
in a variety of atypical appearances on CT [35].
Unfortunately, the technique also involves additional patient
radiation exposure as a baseline scan and two or three post
contrast scans are required for the exam [33]. The dose from a 4D
CT is about 10.4 mSv, compared to 7.8 from a Tc-sestamibi exam,
but the dose to the thyroid is 50 times higher with 4D CT [35].
MRI: On MRI, an adenoma appears as a nodule with a signal intensity similar to muscle or the thyroid on T1 weighted images. On T2 images, the signal intensity is greater and can become isointense with fat. Thus, the typical signal intensity is similar to cervical lymph nodes. A method to help differentiate the two is that adenomas are generally medial to the carotid sheath, whereas lymph nodes are generally lateral to the sheath. The thyroid gland may also demonstrate areas of increased signal on T2 weighted images in up to 40% of patients with no known thyroid disease. With the use of Gd-DTPA, there is very prominent enhancement of parathyroid adenomas which become similar in intensity to fat on the T1 weighted images. Fat suppression techniques will aid in identification of the lesion. Atypical signal characteristics can be seen in 8-30% of cases [15]. MRI has a sensitivity of between 70 to 82% which is similar to scintigraphy [4,15]. When MR findings are combined with Tc-MIBI exam findings, there is a substantial increase in sensitivity and positive predictive value [15]. False positive MR exams can occur with exophytic thyroid nodules and lymph nodes [15]. False negative MR exams can occur in the setting of parathyroid hyperplasia [15]. The sensitivity of MR for the detcetion of parathyroid adenoma is from 46-87% [29].
Scintigraphic Imaging of
Parathyroid Adenomas
Technetium Sestamibi Imaging for Parathyroid Adenomas:
Technetium Sestamibi has been used to localized parathyroid adenomas [5]. Tc-Sestamibi is normally distributed to the parotid and submandibular glands, thyroid gland, heart, and liver [29]. Tracer retention in the arm vein used for the injection is commonly seen [29]. Mild to moderate tracer accumulation can be seen in the oral cavity secondary to secretion from the salivary glands [29]. In younger individuals, mild to moderate thymic uptake may be seen [29]. Uptake may also be seen in supraclavicular brown fat [29]. The normal parathyroid gland is too small to be seen on routine imaging [29]. Either a dual phase or subtraction examination may be performed. Disadvantages of the subtraction exam include the requirement for two radionuclide injections and a cooperative immobile patient- also, there is an increased likelihood for artifacts on images obtained with digital subtraction [29].
1- Dual Phase Exam:
For the dual phase exam, 10 to 20 mCi Tc-sestamibi is given I.V. and images are performed over the neck and chest at 15 minutes, and 2 to 4 hours post injection. Images should be performed over the neck and thorax. SPECT images acquired in a 128x128 matrix can also be performed. Some researchers feel that SPECT images should be performed in all patients to increase the exams sensitivity and to provide definitive localization of the lesion [20,21]. SPECT images are usually acquired at a single time interval- either early or delayed. Early SPECT images may be superior, but the difference may not be statistically significant [25]. For SPECT acquisitions a large field of view dual detector camera with a 20% energy window centered at 140 keV is used with a high-resolution low-energy parallel hole collimator [29]. A 360 degree acquisition is performed using a step-and-shoot protocol for 25 seconds per projection with a 128x128 matrix (zoom factor 1.28) [29]. Images are reconstructed by using a Butterworth prefilter (cutoff, o.5; order 10.0), a Butterworth post filter (cutoff, 0.7; order, 10.0), and an ordered-subset expectation maximization iterative technique (eight subsets, two iterations) [29]. SPECT/CT imaging can provide superior performance to conventional planar imaging (pooled sensitivity of 86% [42]) and more accurate lesion localization, but at the cost of additional patient radiation exposure [28]. Some authors suggest that obtaining both early and delayed SPECT/CT can depict more lesions than with either phase alone [36].
There is increased tracer accumulation within most parathyroid
adenomas on both initial and delayed images. A parathyroid adenoma
that is contiguous with the thyroid gland is detectable on
early-phase images only if it has radiotracer accumulation greater
than that of the thyroid gland or causes an asymmetric bulging of
the thyroid contour [29]. Although usually apparent on early
images, the lesion typically becomes more apparent on later
scanning due to faster clearance of the agent from the thyroid
gland. Prolonged tracer retention within the parathyroid adenoma
is felt by some to be related to the presence of mitochondrial
rich oxyphil cells within the lesion
(remember that Tc-Sestamibi is felt
to localize within mitochondria) [16]. In one series, delayed
washout was noted in 60% of parathyroid adenomas- therefore, a
large percentage of lesions may have early tracer washout [29].
Therefore, any suspicious finding on early phase imaging should be
reported as such a finding may lead the surgeon to explore that
area first [29].
Tc-sestamibi uptake within a thyroid
adenoma or thyroid carcinoma, or a cervical lymph node can produce
a false-positive exam [14]. Focal uptake in a thyroid nodule
should prompt further evaluation as up to 16% of these nodules
have been shown to be malignant [39].
False negative exams occur in patients with parathyroid hyperplasia. When feasible, concomitant suppression of thyroid uptake (induced by exogeneous thyroid hormone administration) can improve localization of parathyroid lesions with Tc-sestamibi [21].
Lesion size/weight plays an important role in scintigraphic detection (sensitivity is lower for smaller lesions) [16,27], but other factors may also influence detection [8]. Some parathyroid adenomas demonstrate rapid release of Tc-sestamibi and may only be apparent on the early images. Rapid tracer washout may be related to a small number of oxyphil cells in the lesion [6]. However, other authors disagree and have found no relationship between lesion detection and predominant cell type [8]. P-glycoprotein is a plasma membrane lipoprotein which is believed to increase the efflux of chemotherapeutic drugs from cancer cells. Sestamibi serves as a substrate for P-glycoprotein transport, however, P-glycoprotein expression within parathyroid adenomas has not been shown to be associated with decreased lesion detection [8].
Overall, dual phase Tc-sestamibi
imaging has a sensitivity of between 45 to 95% (average 73% [7]),
specificity 85-100% [7] and a PPV of 97%. The sensitivity for multigland disease is lower (mean 55%)
[27]. A large meta-analysis found the sensitivity for the
detection of a solitary adenoma to be 88% (CI 87-89%), for
hyperplasia 44% (CI 41-48%), and for double adenomas 30% (CI
2-62%) [24]. Imaging with Tc-Sestamibi detects an additional 10% of
lesions not identified on thallium-pertechnetate
imaging. The surgical failure rate has been reported to be higher
when preoperative imaging findings are negative (as high as 10%)
[37]. Patients with negative ultrasound and nuclear results tend
to have smaller lesions and a higher proportion of hyperplasia (up
to 22%) [37].
In cases of rapid tracer release, Tc-Sestamibi
- I-123 subtraction imaging may be superior to the dual phase
exam. Overall, the dual phase exam is probably less sensitive than
the Tc-sestamibi subtraction exam
[7,37].
Brown fat activity has been described on Tc-sestamibi parathyroid imaging [26].
|
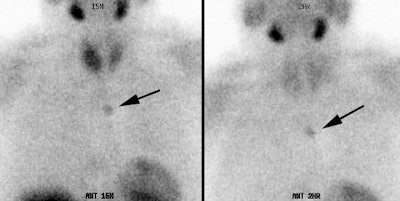
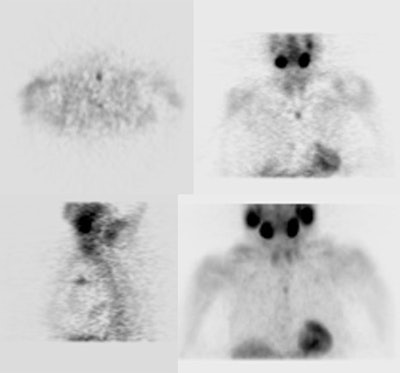
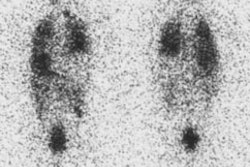
Parathyroid adenoma: Early and delayed Tc-99m-MIBI images of a typical parathyroid adenoma. Note tracer retention within the adenoma. |
|
|
|
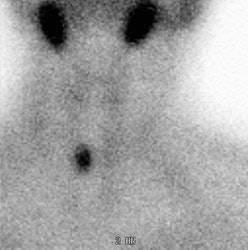
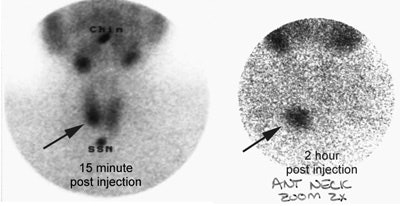
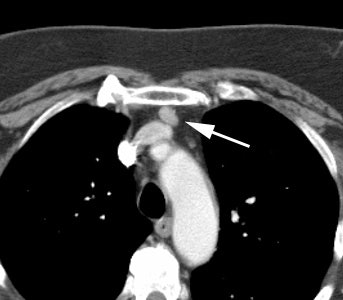
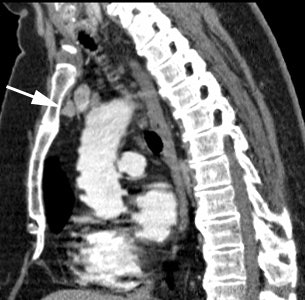
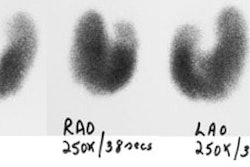
Ectopic parathyroid adenoma: The patient in the case below presented with hyperparathyroidism. A dual phase Tc-MIBI examination revealed a persistent focus of tracer accumulation in the chest (black arrows) on both early and delayed imaging. A SPECT exam was performed to better localize the abnormality which can be seen in the anterior mediastinum (click SPECT images to view cine). CT scan confirmed a soft tissue nodule in the anterior mediastinum (white arrows). At surgery, the patient was found to have an ectopic parathyroid adenoma. |
|
|
|
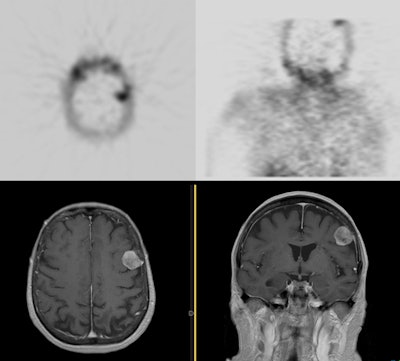
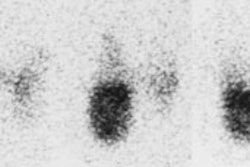
Meningioma on sestamibi parathyroid scan: A Tc-MIBI exam demonstrated a focal area of tracer uptake in the left parietal lobe of the brain. This area was not included on the planar images. As sestamibi is also a tumor agent, a MRI of the rbain was performed and demonstrated a meningioma corresponding to the area of tracer uptake. Click here to view a cine loop of the SPECT MIP images |
|
|
2- Tc-Sestamibi Subtraction Exam:
Rapid tracer washout from parathyroid adenomas can occur in up to 40% of cases and can result in false negative exams [20]. In cases of rapid tracer release, Tc-Sestamibi subtraction imaging may be superior to the dual phase exam. For this exam, either I-123 or Tc-pertechnetate is used to outline the thyroid gland and this image is subtracted from the Tc-Sestamibi image (which would outline both thyroid and parathyroid tissue).
If using Tc-pertechnetate, the pertechnetate exam can be performed first by using a low dose of Tc-pertechnetate (5 mCi) and administering potassium perchorate to achieve a rapid washout of the agent prior to initiation of the Tc-sestamibi portion of the exam [21]. Alternatively, the Tc-pertechnetate exam can be performed following completion of the dual-phase Tc-sestamibi study as most of the agent will have washed out of the thyroid [21].
If using I-123, images can be acquired using simultaneous double-energy window aquisition [19]. Window limits should be set to include both photo peaks, but to diminish overlap as much much as possible. A window limit of 140 keV +/- 7% for Technetium, and 159 keV with a 4% lower limit and 10% upper limit for I-123 are acceptable [10,11]. Pin hole imaging is required for maximum sensitivity [37]. SPECT images can also be obtained simultaneously [32]. The sensitivity of 123I/99mTc-sestamibi subtraction SPECT for the evaluation of hyperparathyroidism is 71% [32] to 94% [37].
Overall, the Tc-sestamibi subtraction exam is probably more sensitive than the dual phase exam for the identification of parathyroid adenomas [7]. The Tc-sestamibi subtraction exam has been shown to have a sensitivity of about 89% in the detection of parathyroid adenomas [7]. The sensitivity ranges from 55 to 100% in the detection of hyperplasia [7,10,11].Uptake in hyperplasitic glands correlates with the weight of the gland [10]. As with thallium-pertechnetate imaging, false positive studies can be seen in patients with cold thyroid nodules. Subtraction imaging should probably not be used in patients with suppressed thyroid function.
Tc-Tetrofosmin:
Tc-tetrofosmin has also been used in
the detection of parathyroid adenomas and hyperplasia using a dual
phase examination technique similar to that for Tc-sestamibi. The overall sensitivity and
specificity have been reported to be 77.3-87% and 83%,
respectively [12,13]. Sensitivity for
detection of adenomas is superior to parathyroid hyperplasia.
Uptake of Tc-Tetrofosmin is
independent of the number of oxyphil
cells within the lesion.
Thallium-Pertechnetate Subtraction Imaging for
Parathyroid Adenomas:
Previous scanning employed the use of technetium pertechnetate and Thallium (Tl) 201. Thallium is a perfusion agent and will be taken up by both the parathyroid adenoma and the adjacent thyroid. Peak parathyroid uptake of Tl-201 occurs between 5 to 10 minutes after injection, and it is essential that images be obtained within the to 30 minutes. Pertechnetate is selectively taken up (trapped) by the thyroid and salivary glands. Pertechnetate and thallium images are collected for the same number of counts. After normalization, the pertechnetate image is then subtracted from thallium image and the remaining activity in the neck theoretically represents the parathyroid glands/adenoma. It is also important to obtain an image over the chest during the thallium exam, as this may identify an ectopic gland.
The detection rate for dual isotope scanning is about 70 to 75%
(similar to MRI). It is better in detecting adenomas than
parathyroid hyperplasia. In general, at least 300 mg of
parathyroid tissue (a gland approximately 1 cm in diameter) is
required for visualization with subtraction scintigraphy.
The ability to detect four gland hyperplasia or tumors less than
0.3 g in size is limited. The most common cause of a
false-positive study is patient motion. The accuracy of the study
is also decreased in patients with concomitant thyroid disease
(i.e.- any cold nodule [thyroid
neoplasm, adenoma/colloid cyst, focal Hashimoto's thyroiditis, mets,
lymphoma, sarcoid], MNG, ) which can
also produce false-positive exams.
PET imaging for Parathyroid Adenomas:
PET imaging using 18F-fluorocholine has shown promise
for the evaluation of hyperparathyroidism. Choline is part of the
phospholipid layer in the cell membrane and it has been
hypothesized that hyperfunctioning parathyroid cells have
increased activity of the phospholipid/calcium positive dependent
protein kinase [40]. This leads to increased choline uptake [40].
The exam has been shown to be superior to conventional nuclear
medicine imaging with a sensitivity 90-95.5% for the detection of
parathyroid adenomas (detecting smaller lesions - median gland
weight 0.4 g - compared to conventional nuclear imaging)
[37,40,42]. 18F-fluorocholine PET is particularly
useful in detecting patients with multiple hyperfunctioning
glands- sensitivity 88%, compared to 22-34% for conventional
imaging [42]
Secondary
Hyperparathyroidism
Secondary hyperparathyroidism occurs in response to chronic hypocalcemia due to some other metabolic
disorder, most commonly chronic renal insufficiency [21]. Renal
impairment results in phosphorus retention (decreased renal
phosphate excretion) that will bind calcium and form insoluable
calcium phosphate and reduced synthesis of calcitriol (the active
form of vitamin D) - these changes lead to hypocalcemia
[21,36]. The hypocalcemia stimulates
PTH release- unfortunately, the tubular calcium sparing mechanism
of the kidneys does not respond due to the underlying renal
dysfunction [21]. Secondary hyperparathyroidism is characterized
by enlarged hyperplastic parathyroid
glands. In patients with hyperplasia, the glands may be quite
small and are frequently not detected with any imaging modality.In
suitable candidtaes, parathyroidectomy can ameliorate HPT symptoms
[36]. However, the rate of persistent and recurrent disease
following parathyroidectomy is high- ranging from 10-30% [36].
MOst failures occur when the surgeon does not remove all
hyperfunctioning parathyroid tissue [36].
Tertiary hyperparathyroidism:
Tertiary hyperparathyroidism is seen in cases of secondary hyperthyroidism and occurs when the hyperparathyroidism persists following renal transplant (or correction of the initial cause) due to the development of functional autonomy in an enlarged (over 1 cm) parathyroid gland [21,23]. This results in hypersecretion of PTH in the setting of normal calcium levels [23].
Familial hypercalcuric hypercalcemia:
Patients with this disorder have abnormally elevated levels of calcium and PTH [30]. However, analysis of a 24 hour urine collection will reveal abnormally low levels of calcium [30]. Surgery is not indicated in patients with this disorder [30].
REFERENCES:
(1) AJR 1998; Lane MJ, et al. Use of color and power doppler sonography to identify feeding arteries
associated with parathyroid adenomas. 171: 819-823
(2) AJR 1996;166: 1465-70
(3) AJR 1998; Lane MJ, et al. Use of color and power doppler sonography to identify feeding arteries associated with parathyroid adenomas. 171: 819-823
(4) AJR 1996, 166:705-710
(5) J Nucl Med, 1992; Taillefer R, et al. Detection and localization of parathyroid adenomas in patients with hyperparathyroidism using a single radionuclide imaging procedure with technetium-99m-sestamibi (Double phase study). 33: 1801-07
(6) J Nucl Med, Feb. 95, p.241-43
(7) Semin Nucl Med 1995; McBiles M, et al. Sestamibi parathyroid imaging. 25: 221-34
(8) J Nucl Med 1998; Bhatnagar A, et al. Technetium-99m-sestamibi parathyroid scinitgraphy: Effect of p-glycoprotein, histology and tumor size on detectability. 39: 1617-1620
(10) Radiology 1998; Jeanguillaume C, et al. Secondary hyperparathyroidism: Detection with I-123-Tc-99m-Sestamibi subtraction scintigraphy versus US. 207: 207-213
(11) J Nucl Med 1998; Hindie E, et al. Parathyroid imaging using simultaneous double-window recording of technetium-99m-sestamibi and iodine-123. 39: 1100-1105
(12) J Nucl Med 1998; Ishibashi M, et al. Comparison of Technitium-99m-MIBI, technetium-99m-tetrofosmin, ultrasound, and MRI for localization of abnormal parathyroid glands. 39: 320-324
(13) J Nucl Med 1997; Ishibashi M, et al. Localization of parathyroid glands using technetium-99m-tetrofosmin imaging. 38: 706-711
(14) Radiographics 1999; Nguyen BD. Parathyroid imaging with Tc-99m sestamibi planar and SPECT scintigraphy. 19: 601-614
(15) Radiology 2001; Gotway MB, et al. Comparison between MR imaging and 99mTcMIBI scintigraphy in the evaluation of recurrent or persistent hyperparathyroidism. 218: 783-790
(16) Eur J Nucl Med 2001; Melloul M, et al. 99mTc-MIBI scintigraphy of parathyroid adenomas and its relation to tomour size and oxyphil cell abundance. 28: 209-213
(17) J Nucl Med 1992; O'Doherty MJ, et al. Parathyroid imaging with technetium-99m-sestamibi: Preoperative localization and tissue uptake studies. 33: 313-318
(18) Radiology 2001; van Dalen A, et al. Minimally invasive surgery for solitary parathyroid adenomas in patients with primary hyperparathyroidism: Role of ultrasound with supplemental CT. 220: 631-639
(19) J Nucl Med 2002; Rink T, et al. Limited sensitivity of parathyroid imaging with 99mTc-sestamibi/123I subtraction in an endemic goiter area. 43: 1175-1180
(20) J Nucl Med 2003; Lorberboym ML, et al. Incremental diagnostic value of preoperative 99mTc-MIBI SPECT in patients with a parathyroid adenoma. 44: 904-908
(21) J Nucl Med 2003; Mariani G, et al. Preoperative localization and radioguided parathyroid surgery. 44: 1443-1458
(22) J Nucl Med 2005; Rubello D, et al. Radioguided surgery of primary hyperparathyroidism using the low dose 99mTc-sestamibi protocol: multiinstitutional experience from the Italian study group on radioguided surgery and immunoscintigraphy (GISCRIS). 46: 220-226
(23) Radiographics 2005; McDonald DK, et al. Primary hyperparathyroidism due to parathyroid adenoma. 25: 829-834
(24) AJR 2007; Johnson NA, et al. Parathyroid imaging: technique and role in the preoperative evaluation of primary hyperparathyroidism. 188: 1706-1715
(25) J Nucl Med 2007; Lavely WC, et al. COmparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase 99mTc-sestamibi parathyroid scintigraphy. 48: 1084-1089
(26) J Nucl Med 2008; Goetze S, et al. Visualization of brown adipose tissue with 99mTc-methoxyisobutykisonitrile on SPECT/CT. 49: 752-756
(27) Radiology 2008; Nichols KJ, et al. Preoperative parathyroid scintigraphic lesion localization: accuracy of various types of readings. 248: 221-232
(28) J Nucl Med 2008; Buck AK, et al. SPECT/CT. 49: 1305-1319
(29) Radiographics 2008; Eslamy HK, Ziessman HA. Parathyroid scinitgraphy in patients with primary hyperparathyroidism: 99mTc-sestamibi SPECT and SPECT/CT. 28: 1461-1476
(30) J Nucl Med 2008; Judson BL, Shaha AR. Nuclear imaging and minimally invasive surgery in the management of hyperparathyroidism. 49: 1813-1818
(31) AJR 2008; Veldman MW, et al. Percutaneous parathyroid ethanol ablation in patients with multiple endocrine neoplasia type I. 191: 1740-1744
(32) J Nucl Med 2008; Neumann DR, et al. Preoperative 123I/99mTc-sestamibi subtraction SPECT and SPECT/CT in primary hyperparathyroidism. 49: 2012-2017
(33) AJR 2011; Beland MD, et al.
Dynamic MDCT for localization of occult parathyroid adenomas in 26
patients with primary hyperparathyroidism. 196: 61-65
(34) J Nucl Med 2013; Schalin-Jantti C, et al. Planar
Scintigraphy with 123I/99mTc-Sestamibi, 99mTc-Sestamibi SPECT/CT,
11C-Methionine PET/CT, or Selective Venous Sampling Before
Reoperation of Primary Hyperparathyroidism? 54: 739-747
(35) Radiology 2014; Hoang JK, et al. How to perform parathyroid
4D CT: tips and traps for technique and interpretation. 270:
15-24
(36) AJR 2014; Yang J, et al. Value of dual-phase 99mTc-sestamibi
scintigraphy with neck and thoracic SPECT/CT in secondary
hyperparathyroidism. 202: 180-184
(37) J Nucl Med 2015; Hindie E, et al. The role of radionuclide
imaging in the surgical management of primary hyperparathyroidism.
56: 737-744
(38) Radiology 2015; Bahl M, et al. Parathyroid adenomas and
hyperplasia on four-dimensional CT scans: three patterns of
enhancement relative to the thyroid gland justify a three-phase
protocol. 277: 454-462
(39) AJR 2016; Yerubandi V, et al. Incidental thyroid nodules at
non-FDG PET nuclear medicine imaging: evaluation of the prevalence
and malignancy rate. 206: 420-425
(40) Radiology 2017; Kluijfhout WP, et al. 18F
fluorocholine PET/MR imaging in patients with primary
hyperthyroidism and inconclusive conventional imging: a
prospective pilot study. 284: 460-467
(41) Radiology 2019; Yeh R, et al. Diagnostic performance of 4D
CT and sestamibi SPECT/CT in localizing parathyroid adenomas in
primary hyperparathyroidism. 291: 469-476
(42) J Nucl Med 2020; Cuderman A, et al. 18F-fluorocholine
PET/CT in primary hyperparathyroidism: superior diagnostic
performance to conventional scintigraphic imaging for localization
of hyperfunctioning parathyroid glands. 61: 577-583
(43) Radiographics 2020; Bunch PM, et al. Parathyroid 4D CT: what
the surgeon wants to know. 40: 1383-1394