Thyroid Neoplasms
Thyroid Carcinoma
The incidence of thyroid cancer continues to rise with a 2.4 fold
increase in incidence since 1975 [30]. As of 2002, the prevalence
of thyroid cancer in the US was approximately 9 per 100,000, or
24,000 new cases per year [22]. Thyroid cancer accounts for about
1.5% of cancers diagnosed in the US [28]. About 10% of patients
with differentiated thyroid cancer have distant metastases at
presentation or develop distant metastases during followup [47].
If the metastases can be completely cured by radioiodine therapy,
the overall 10 year survival is 92%, compared with 29% in patients
with residual disease [47].
The thyroid gland primarily compromises follicular cells and C-cells along with the necessary supporting stroma [37]. Medullary thyroid cancer arises from the c-cells (which secrete calcitonin and take part in calcium homeostasis) [37]. The hallmark of follicular cells is the active uptake of iodine from the bloodstream with subsequent organification [37]. Differentiated thyroid cancer arises from follicular cells and can be divided into papillary (most common), follicular, and Hurthle cell subtypes [22,37]. Thyroid cancer can occur at any age, but has a bimodal distribution peaking in the 3rd and 6th decades [22]. Women are affected more than men (3:1) [22]. Previously, most patients with thyroid cancer presented with a palpable nodule, however, occult cancers are being increasingly detected by imaging studies performed for other reasons [22].
Imaging
Thyroid carcinomas almost invariably appear as cold areas on routine thyroid scanning. In general, it is estimated that thyroid tumors will accumulate 0.01 to 0.02% of the injected dose of I-131 per gram. When contrasted with the normal thyroid which accumulates 0.5 to 1.0% of the injected dose per gram, it becomes obvious why carcinomas appear as cold nodules within the intact thyroid, but appear hot on post thyroidectomy/ablation scans. Most thyroid carcinomas appear hypoechoic (65%) or isoechoic (25%) on ultrasound. Hyperechoic thyroid lesions tend to be benign (95%). Only 2% of thyroid carcinomas appear as cystic lesions. Calcifications can be found in both benign and malignant nodules (particularly medullary carcinoma).
Ultrasound has been explored for the staging of differentiated thyroid cancer [23,24]. The overall accuracy of US for N staging is 71% ( sensitivity 51-62%, but much higher for the lateral compartments; specificity 79-98%) [23,24]. Criteria for abnormal lymph nodes on US include absence of an echogenic hilum, a rounded shape (ratio of long axis to short axis <1.5), minor axis gretaer than 10 mm, minor axis greater than 50% of the major axis, focal or diffuse hyperechoic change of node versus adjacent muscle, calcification, cystic change, and a chaotic or peripheral color doppler pattern [23,24]. The overall sensivity for US in determining T-stage is about 67% [23]. Various US criteria have been used to determine extrathyroid extension including >50% of the tumor abutting the thyroid capsule (other authors have used contact with the adjacent thyroid capsule along more than 25% of the perimeter of the tumor [24]), and loss of echogenic thyroid capsule at the contact site of the primary tumor (i.e.- no thyroid tissue intervening between the primary tumor and the capsule), [23,24]. The sensitivity for US in detecting extrathyroid extension is 85%, with a specificity of 70%, and an accuracy of 74.5% (using the criterion of >50% of the tumor abutting the thyroid capsule) [23].
Risk factors for a thyroid mass being cancer include:
1- Male sex
Two fold increased risk; however, females have an overall higher incidence of thyroid cancer as they have 8 times as many thyroid nodules as men [9]. Overall, women are 3 times more likely to develop thyroid cancer than men [28].
2- Age
Under 20 or over 60 years [14]. Some authors recommend that in males over the age of 60 years, the pretest probability of a thyroid nodule being cancer is so high that surgery should be considered even if the fine needle biopsy results are negative [9].
3- History of Radiation Therapy to the Head and Neck
There is clear evidence that pediatric patients exposed to low dose radiation of the thyroid are at increased risk for developing thyroid carcinoma, as well as benign thyroid nodules. Within the intermediate exposure range of 100 to 200 rads, the rate of radiation induced thyroid carcinoma increases in a linear manner up to a dose of about 1500 rads. Above this level, there is a steep decrease in the incidence of thyroid cancer and a progressive increase in the incidence of hypothyroidism most likely due to destruction of the gland at the higher doses [1]. Carcinoma is most likely to result following exposure during early childhood [12]. The peak risk is seen 5-30 years post radiation. Thyroid carcinoma is identified in about 30% of these patients. XRT is primarily associated with an increased risk for papillary carcinoma, and to a lesser degree follicular carcinoma. Besides thyroid malignancies, other thyroid abnormalities are seen in 20% of patients including adenomatous hyperplasia/follicular adenoma (70%).
4- Family history of thyroid cancer [22]
A follicular adenoma is a benign proliferation of follicles surrounded by a complete capsule [34]. When a nodule is reported as a "follicular neoplasm" at FNA, there is a 70-85% chance of it being a follicular adenoma (and a 15-30% risk for malignancy) [34].
Papillary Thyroid Carcinoma (Roughly 66-80% of thyroid cancers)
The term papillary carcinoma of the thyroid describes both pure
papillary tumors and those lesions that contain both papillary and
follicular elements ("mixed" tumors) [6]. Papillary carcinoma is
the most common thyroid cancer accounting for 50-89% of cases
[4,6,14,22,34]. Small papillary cancers have been found in 6-13%
of American patients in autopsy series [4]. Females are affected
more commonly than males. The mean age for patients to present is
about 45. The majority of tumors are unilateral (70-80%), but can
be multifocal in up to 25% of patients. Extrathyroidal extension
is found in about 15% of patients. Coexistent benign nodules are
found in 33% of patients [4]. Chronic lymphocytic thyroiditis is
found in about 20% of patients and Graves' disease in about 4%
[4]. Papillary cancer can be multifocal in 26-32% of patients and
can be bilateral in almost 20% [4].
In general, papillary cancers tend to be slow growing and there
is about 93-97% long term survival (25 years) in patients who have
complete surgical resection of the tumor and no evidence of
metastatic disease. This is significantly better than survival in
patients with follicular thyroid cancer [4]. However, in patients
with differentiated thyroid cancer, mortality increases to 25-45%
at 10 years for patients with stage II-IV disease [27]. Certain
histologic cell types of papillary cancer have a worse prognosis -
specifically tall cell variant, columnar cell variant, and diffuse
sclerosing variant [30]. I-131 uptake in metastatic foci during
radioiodine therapy also has an effect on prognosis [32]. The 10
year survival in patients with well-differentiated thyroid cancer
that showed I-131 uptake after therapy was 56%, versus 10% in
patients without I-131 uptake [32].
Prognostic factors associated with an increased mortality:
* Distant metastases present at time of diagnosis [45 fold increase in mortality]. The presence of initial distant metastases is the most potent prognostic factor for survival [4]. Distant metastases are found in 2-7% of patients at initial diagnosis [4]. Mortality at 10 years approaches 70% in this subgroup of patients [4].
* Age over 45-50 years [32 fold] with mortality rates increasing with age
* Primary lesion larger than 2 cm [6 fold](other authors
indicate size greater than 4 cm or high TNM stage at
presentation [39])
* Local/Extrathyroidal invasion [6 fold] - extrathyroidal tumor extension is associated with an increased risk of tumor recurrence and increased cancer-specific mortality [20]
* Male sex [2 fold]
* Increased grade of the primary tumor (grade greater than or equal to 2) [4]
* Angioinvasion is seen in only about 2% of cases and is associated with a worse prognosis
* Tumor genetics:
The presence of BRAF mutation confers a risk for development of
thyroid cancer of nearly 100% in the setting of indeterminate
cytologic findings [43]. The V600E BRAF mutation is the most
common genetic alteration in papillary thyroid cancer
(identified in 33-73% of cases of PTC) and is associated with
more aggressive behavior, development of radioiodine refractory
recurrent disease through suppression of key genes involved in
radioiodine uptake, and poor clinical outcome [22,33,39,47,48].
Up to 76% of patients with the BRAF V600E mutation have been
found to have lymph node metastases at the time of initial
surgery, compared to 17% of V600E-negative patients [22].
Studies have identified this mutation as an independent
predictor of tumor recurrence [22]. The V600E mutation has also
been associated with increased cancer related mortality in
patients with PTC, increased risk for extrathyroidal invasion,
decreased I131 positivity, and poor treatment response to
radioiodine/decreased radioiodine avidity in distant metastatic
disease [22,33,42]. BRAF V600E mutated tumors are also more
FDG avid due to an increased expression of glucose transporter 1
[38]. When both BRAF V600E and TERT mutations are both present
they are robustly associated with poor clinical outcomes in
patients with papillary thyroid cancer and loss of RAI avidity
[48].
Another mutation found in papillary thyroid cancer involves the
receptor tyrosine kinase (RET) [22]. An RET mutation has been
found in 20-40% of adult papillary thyroid cancer patients [22].
The RET mutation has an increased prevalence in patients with
prior radiation exposure, with tumors manifesting in childhood
and young adults, and with tumors demonstrating a greater
propensity for development of metastases [22].
RAS mutations are found in approximately 10-20% of PTCs and
40-50% of FTCs, and are also responsible for gene suppressions
that result in radioiodine refractory disease [47].
Telomerase reverse transcriptase (TERT) promoter mutation has
also been reported to be associated with aggressive
characteristics in differentiated thyroid cancer [42]. TERT
mutation is associated with older age at diagnosis, male sex,
larger mean tumor diameter, extrathyroidal invasion, vascular
invasion, distant metastases, and greater likelihood for
coexistent BRAF mutation (coexistence of TERT and BRAF mutations
form a genetic background that defines PTC with the worst
clinical outcomes) [42]. Additionally, patients with distant
metastatic DTC with TERT mutation are more likely to be, or
become, radioiodine refractory [42]. However, another article
suggested that the TERT mutation alone is not associated with an
increased risk for iodine refractory recurrent disease, but that
the combination of TERT and BRAF V600E is strongly associated
with loss of RAI avidity [48]. The prevalence of the TERT
mutation varies significantly among different
countries/populations- ranging from 4.1-25.5% for PTC and from
5.9-36.4% for follicular thyroid cancer [42].
Recurrent thyroid cancer:
The American Thyroid Association Guidelines Task Force stratifies
the risk of tumor recurrence:
1- Low risk: absence of local or distant metastatic disease, absence of extracapsular or vascular invasion by the primary tumor, complete surgical resection, non-aggressive histologic characteristics, and negative I-131 whole body imaging
2- Intermediate risk: microscopic invasion of perithyroid soft tissues, aggressive histologic features (tall cell, insular, columnar subtypes), or microvascular invasion
3- High risk: macroscopic extracapsular tumor invasion, incomplete tumor resection, or distant metastases [22]. Recurrence is most commonly seen in the anterior cervical lymph nodes or thyroid bed and most commonly occurs within the 1st decade after treatment [22].
Local recurrence is defined as histologically confirmed tumor in the resected thyroid bed, thyroid remnant, or adjacent tissues of the neck [4]. It is associated with an increase in mortality. It occurs in about 5-15% of patients and is associated with an approximately 40% mortality. The incidence of local recurrence is higher in children, in patients over age 60 years, and in patients initially noted to have extrathyroidal invasion or very large tumors (over 4 cm) [4]. The presence of nodal mets at the time of diagnosis does not increase the risk for local recurrence, but does increase the risk for subsequent development of other cervical nodal mets and distant mets (see below) [4]. Recurrence is more common in patients who have had lobectomy (11%), as compared to those who had total thyroidectomy (4%). Radioiodine therapy has been shown to decrease local recurrence by about 50%, but unfortunately, therapy has not been shown to statistically decrease mortality in patients with thyroid cancer.
Distant Metastatic Disease in Papillary Carcinoma:
Lymph nodes:
Lymphangitic spread to local neck nodes is most commonly seen
and such regional nodal metastases are found in 20 to 50% of
patients at the time of diagnosis [15], even with small
intrathyroidal tumors (microcarcinomas) [30]. The cumulative
risk of developing lymph node metastases increases continuously
with a primary tumor diameter of 5 mm or greater [30]. The
prevalence of nodal metastases in PTC has been reported to be
13-55% for tumors less than 5 mm in diameter, and 59-74% for
those measuring 5-10 mm [39]. Nodal metastases are separated
into central compartment (level IV) nodes - N1a, versus
lateral-compartment or superior mediastinal lymph node
metastases (N1b) [22]. A minimum axial diameter of 7 mm for
level II cervical nodes and 6 mm for the rest of the cervical
nodes has been shown to have high sensitivity (93%) and
specificity (83%) for detection of metastatic lymph nodes [39].
The presence of initial local nodal mets does NOT influence
survival, but does increase the risk for recurrence [6].
However, other authors indicate that an increase in the number
of metastatic lymph nodes is associated with a decrease in
survival (with up to 6 metastatic nodes) [46]. About 7% of
patients will subsequently develop nodal metastases, typically
within 5 years [4]. The risk for subsequent nodal metastases is
increased when patients had metastatic nodes at presentation,
the patients are under age 20 years, the tumor size was greater
than 4 cm, and if there was the presence of extrathyroidal
invasion at the initial surgery [4].
Pre-operative US evaluation of the neck can identify suspicious cervical adenopathy in 20-33% of cases and this can potentially alter the surgical approach [15,22]. Pre-operative neck US is recommended for all patients with thyroid cancer prior to thyroidectomy [15]. Features on US that suggest pathologic lymph nodes include echogenic nodes (up to 80% of metastatic PTC nodes can appear echogenic [39]), cystic change/cystic necrosis, cmicrocalcification/alcification, and loss of the normal nodal architecture [34,39]. At the time of initial surgery, routine central-compartment (level IV) lymph node dissections are commonly performed [22]. Although the survival benefit of routine node dissection is controversial, local recurrence to central compartment nodes is common and the likelihood for injury to the recurrent laryngeal nerve is increased if repeat surgery is performed [22].
Distant metasases- General:
Distant (hematogenous) mets represent a rather uncommon event in differentiated thyroid cancer (either papillary or follicular). Distant mets are seen in only about 3-7% of patients with papillary carcinoma at the time of diagnosis. Approximately 4-5% of patients will go on to develop distant mets at some time, generally within the first 10 years after surgery [4]. The most common site for distant mets is the lungs, followed by bone and the mediastinum [4,22]. Factors associated with an increased risk for subsequent distant metastases include a tumor size greater than 4 cm, extrathyroidal invasion, age over 60 years, tumor grade greater than or equal to 2, and the presence of metastatic cervical nodes at the time of initial surgery [4].
The most common site for distant metastatic disease is the lung [22]. Overall, men have a higher incidence of pulmonary mets. Between 50-60% of metastatic pulmonary lesions will concentrate radioiodine [6]. Patients with iodine concentrating pulmonary metastases have a 5-year survival rate of 60% compared to 30% for tumors which do not concentrate radioiodine [13]. The early (pre-radiographic) scintigraphic diagnosis and I-131 therapy of lung mets appears to be the most important element in obtaining both a significant improvement in survival rate and a prolonged disease free time interval in these patients. In patients with a negative CXR, but positive I-131 scans, there was a 10 year survival rate of 96% and a complete disease remission rate of 80% following I-131 therapy. In patients with both a positive CXR and a positive I-131 scan, the 10 year survival rate was significantly lower (40%) and complete remission was rarely achieved (4%). Patients with a positive CXR, but a negative I-131 scan had the worst prognosis with a 10 year survival rate of only 10% and no complete remissions. In summary- the presence of iodine concentrating pulmonary mets with a negative CXR appears not to influence mortality, however, non-concentrating micro- or macronodular mets do increase mortality risk.
Pulmonary metastases may appear on CT as micronodules (less than 5 mm), macronodules, or the lesions may be radiographically occult and only identified following I-131 imaging or treatment [22]. The micronodular pattern (miliary) of lung mets is invariably related to good I-131 uptake and a better prognosis, while macronodular (over 0.5 cm) mets frequently showed poor uptake and had an associated worse prognosis (fatal outcome was almost always observed in patients with macronodular mets and no I-131 accumulation). Reported 5 year survival rates are 100% for CT occult disease, 88% for micronodules, and 25% for macronodules [22].
|
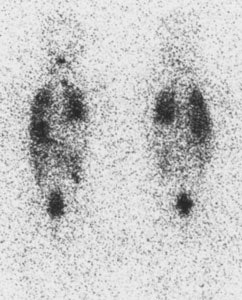
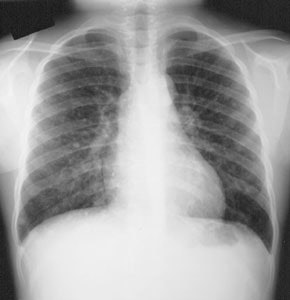
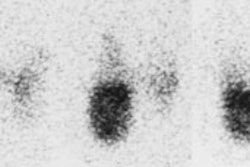
Diffuse lung metastases: The patient was an 8 year old female with thyroid cancer. The I-131 exam demonstrated diffuse pulmonary tracer accumulation consistent with metastatic disease. The CXR demonstrated multiple small pulmonary nodules (Click CXR to enlarge image) |
|
|
The presence of mets in distant sites other than the lungs (bone, liver, brain) is an unfavorable prognostic variable in patients with documented lung mets. Tumor histology in lung mets was also noted to have prognostic value, with papillary tumors having a better prognosis than follicular. Patient sex and cervical nodes did not affect prognosis. [5]
Bone metastases are uncommon in patients with differentiated
thyroid cancer and are usually seen after the appearance of lung
mets. In one study, bone metastases at presentation were found
in only 3.5% of patients [7]. Solitary lesions are seen in
30-50% of cases, but more typically multiple lesions are present
(70%) [6,22]. The most common site for bone mets is the spine.
Thyroid mets to bone are best detected by whole body I-131
scans. Only 60% of bone lesions detected on the iodine scan are
identified on bone scan (this is likely because most bone mets
are osteolytic [22]). Bone mets are characteristically
insensitive to I-131 therapy, and up to 50% of lesions will
demonstrate no response to I-131 treatment, although patients
may experience pain relief. External XRT may be beneficial when
the lesions fail to concentrate iodine. The 10 year survival
rate with bone mets is about 45%. [6] However, other authors
have found a very good response to I-131- especially in younger
patients (under age 45 years) and in patients with fewer bone
lesions (less than 3) [7].
Molecular targeted
therapy: Traditional cytotoxic chemotherapy has limited
efficacy in the treatment of metastatic thyroid cancer [39].
Targeted therapies such as sorafenib and lenvatinib have been
approved for the treatment of RAI-refractory metastatic
differentiated thyroid cancer [39,40]. Both agents are multiple
tyrosine kinase inhibitors targeting VEGFR, PDGFR, and RET
[39,40]. Sorafenib also inhibits B-Raf ) the protein product of
BRAF), although less potently than other targets [39].
Follicular Thyroid Cancer (10-20% of thyroid cancers)
Follicular cacinoma accounts for 8-32% of differentiated thyroid
cancers [10,22]. Follicular carcinoma tends to occur in a slightly
older age group (50 years) than papillary cancer and have an
overall worse survival rate [10]. The lesion is multifocal in 10
to 25% of cases and angioinvasion is found in about 50% of cases.
Point mutations of the RAS oncogene are seen in approximately
40-53% of acses of FTC [39]. PPARG (peroxisome
proliferator-activated receptor gamma) rearrangement is also seen
in about 60% of cases [39].
Hematogenous distant metastases are seen in 10-15% of patients at
the time of diagnosis (although other authors state FTC has a
greater tendency to spread hematogenously and distant mets can be
found in 21-33% of patients [39]). Subsequently, distant
metastases will be occur in up to 25% of patients. The most common
location for metastases is the lungs (50-70%), followed closely by
the bones (25-65%), and also the brain (20%). Regional nodal
metastases are found in only about 10% of patients (metastatic
nodes in FTC are usually non-calcified and lack cystic changes,
presenting as solid, homogeneous, and hypoechoic lesions with
peripheral vascularity) [39]). Metastatic lesions are found to be
functioning in about 50% of cases and may be associated with
hyperthyroidism if there is extensive metastatic disease. The
overall mortality from follicular thyroid cancer is about 20%,
however, patients with no evidence of disseminated disease at
presentation have a 10 year survival of about 85-90% [10,34].
Factors associated with a worse prognosis include no
post-operative radioiodine ablation therapy, extrathyroidal tumor
extension/capsular invasion/vascular invasion, disseminated
disease at time of presentation, older age at presentation (>45
years), male sex, presence of an RAS mutation, and post-operative
locally recurrent disease [10,39]. Patients with only minimally
invasive follicular carcinoma have an excellent prognosis [25].
Follicular neoplasms will usually concentrate technetium
pertechnetate, but may not concentrate I-131- producing a
"discordant nodule". Treatment with radioiodine following
thyroidectomy has been shown to decrease the risk of local
recurrence and improve patient survival [10].
Follicular thyroid cancer genetics: RAS mutations are common, as well as PAX8-PPAR-γ (seen in 30-40% of follicular thyroid cancers) [43]. RAS mutations can be seen in follicular adenomas, but RAS-mutated adenomas have higher rates of malignant degeneration and the presence of this mutation may increase the risk for cancer as much as 85% [43].
Thyroid Cancer and Pregnancy
Iodine thyroid scans and therapy are contraindicated during pregnancy. If a thyroid cancer is detected during pregnancy, then surgery may be performed (surgery may be delayed if the lesion is detected late during the pregnancy). Pregnancy has no effect on the natural course of thyroid cancer.
Pediatric Thyroid Cancer
Less than 2-10% of papillary and follicular thyroid cancers occur
in pediatric patients (age less than 20y) [2,35] with about 2/3's
of those affected being female and adolescents affected 10-fold
more commonly than younger children [35]. Disseminated disease is
common- at presentation, regional cervical nodal metastases can be
seen in 60-80% of patients [36]. The presence of extrathyroidal
extension and lung mets are also more common in children than in
adults. The prevalence of lung metastases caries from 5% to 20%
[3]. However, distant metastases do not appear to be a factor in
predicting as poor an outcome as they do in adult patients
[2,3,36]. Mortality for children presenting with distant
metastases is about 15% at 15 years (compared to almost 70% for
similarly affected adults) [2]. Although complete remission of
pulmonary metastases may be difficult to achieve (17-83% of
patients [3]), a partial response is possible with generally good
quality of life, no disease progression, and a low mortality rate
[3].
The therapeutic approach to thyroid cancer in children is identical to that in adults- it includes surgery, radioactive iodine ablation, and TSH suppression with exogenous thyroxine [2]. The use of RAI in pediatric patients has been reported to be safe with no adverse effects on subsequent fertility, pregnancy, or secondary malignancy [35], however, other authors report that decreased fertility may be seen in females that are long-term survivors of childhood thyroid cancer [2].
Following treatment, recurrence has been reported in about 19-24%
of patients [36]. Tumor recurrence in either the thyroid remnants
or cervical nodes is more common than in adults [2,36]. Cervical
nodal metastases can develop in up to 30% of patients [2]. Factors
associated with am increased risk for recurrence include younger
age (especially under age 10 years), less radical primary surgery
(surgery less than total thyroidectomy) without subsequent I131
therapy, and tumor multifocality [36]. In one study, there was no
correlation between lymph node metastases at presentation and risk
of recurrence [36].
Despite the frequent presence of disseminated disease at
presentation, the overall prognosis for pediatric thyroid cancer
is very good [35]. Irrespective of tumor size, multifocality, and
the extent of lymph node involvment, younger patients are
classified as having stage I disease in absence of distant
metastases [35]. The 5 year survival rates for adolescents
with thyroid cancer range from 97.5 to 99.6% [35]. Overall 15-20
year survival for pediatric thyroid cancer patients is 90-95%
[6,36].
Hurthle cell carcinoma
Hurthle cell carcinoma is a follicular variant and it accounts for less than 10% of thyroid carcinomas (about 3% [30]. The tumor is composed of large oxyphilic (Hurthle) cells which contain abundant mitochondria. Most do NOT accumulate radioiodine (non-functional), but are capable of synthesizing thyroglobulin. The tumors tend to invade locally (40%) and recur. The risk for distant metastases is about 33%, compared to 22% for follicular cancer and 10% for papillary cancer [11]. Metastases can be either lymphatic (LN mets are present in 30%) or hematogenous. These patients have an overall worse prognosis with a 5 year survival of 76-81% (Papillary 94%, Follicular 87%) [11,34] and a 10y survival of about 65% (Papillary- 95%, Follicular-85%). However, other authors indicate that there is no significant mortality difference between follicular carcinoma and the Hurthle cell variant [25].
On scintigraphy, both Thallium and Tc-Sestamibi have been shown to localize in recurrent tumor. FDG PET imaging is very good for imaging Hurthle cell neoplasms as intense tracer uptake is generally seen [11].
Anaplastic/Poorly Differentiated: (2-5%)
Anaplastic carcinoma is an undifferentiated neoplasm that
maccounts for only 2% of all primary thyroid malignancies and it
is usually seen in older patients (60-70y). It is the most
aggressive type of thyroid cancer and has the highest prevalence
of distant metastases, occurring in 43% of patients at
presentation [39]. The most common sites of metastatic disease are
the lungs (78%), mediastinal lymph nodes (58%), adrenal glands
(24%), liver (20%), and brain (18%) [39]. Cervical nodal mets can
be seen in 40% of patients and the nodes appear necrotic in about
50% of patients [39]. The lesions typically do not concentrate
iodine and the prognosis is poor with a median survival of 5
months [34]. Poor prognostic indicators include older age at
diagnosis, male sex, tumor more than 6 cm, and cervical nodal mets
at presentation [39].
Medullary Thyroid Carcinoma
Medullary thyroid carcinoma (MTC) arises from the parafollicular
C-cells (derived from neural crest [17]) and accounts for only 1
to 5% of all thyroid malignancies. The mean age at presentation is
60 y. These tumors may actively secrete calcitonin- a hormone
involved in calcium homeostasis. About 80% of cases are sporadic
and the remaining 20% of cases are associated with MEN syndromes
[17]. Specifically, MTC is associated with MEN syndrome IIa (MTC,
hyperparathyroidism, pheochromocytoma) and MEN IIb (MTC, mucosal
neuromas, pheochromocytoma) [16] and is frequently multifocal in
these patients. Metastases occur early to regional lymph nodes
(50-80%) and distant metastases to the liver (most common site-
49-62% of cases and usualy hypervascular), lung, and bone occur in
15-25% [17,39] and less frequently to the brain and skin [26]. The
prevalence of nodal mets is related to tumor size and can be seen
in 20-30% of patients with tumors less than 1 cm, and in 90% of
patients when the tumor is more than 4 cm [39].
Surgery with total thyroidectomy and extensive lymph node dissection is the only effective curative treatment [18].
Calcitonin is the most sensitive marker and typically increases
long before imaging findings appear [29]. Persistent or increasing
serum calcitonin and CEA levels imply residual or recurrent
disease [29]. Between 20-40% of MTC patients have persistent
disease as indicated by increased calcitonin concentrations after
primary surgery, and 10% with undectable post-operative calcitonin
will have occult disease and develop recurrence later [29,41]. The
American Thyroid Association recommends additional imaging in
patients with a post surgical calcitonin level of 150 pg/mL or
greater (including neck US, neck and chest CT, contrast enhanced
liver CT or MR, and spine and pelvic bone MRI) [29,41]. Affected
patients have a 10-year relative survival rate of 75% [34].
However, the calcitonin doubling time is an independent prognostic
factor [31]. Patients with calcitonin doubling times of less than
6 months are a poor prognostic group with only a 25% 5-year
survival [31]. Patients with a calcitonin doubling time of greater
than or equal to 6 months, but less than 2 years (intermediate
risk) have a 92% 5 year survival [31].
Medullary tumors may have 2 components: C-cells and follicular
cells. Occasionally, the lesions may concentrate radioiodine and
this is thought to be due to trapping within the follicular
element. The treatment of choice for medullary thyroid carcinoma
is surgical total thyroidectomy. I-131 therapy does not
significantly affect prognosis in patients with disease outside
the thyroid bed, but may be helpful in decreasing recurrence in
patients with residual foci in the thyroid bed.
Medullary cancer genetics:
Germline mutations activating the RET proto-oncogene are found in
88-95% of hereditary cases and in 40-50% of cases of sporadic MTC
[39]. Mutation in RET results in constitutive activation of the
RET tyrosine kinase receptor [43]. The type of mutation in RET
determines the biologic behavior of MTC [39]. Mutations in the
codons 883, 918, or 928 are associated with an aggressive course,
mutations in codons 768 or 790 are associated with a less
aggressive course [39]. Other factors associated with a poor
prognosis include age over 40 years, poor immunostaining for
calcitonin, high CEA level, and persistent hypercalcitoninemia
after thyroidectomy [39].
Molecular targeted therapies(tyrosine kinase inhibitors) have
been approved for the treatment of metastatic MTC and include
vandetanib and cabozantinib [39,44]. Vandetanib is a TKI targeting
VEGFR, RET, and EGFR pathways [39]. The agents are associated with
increased progression free survival and overall survival in
patients with RET-mutated MTC [43,44].
On US, punctate echogenic foci due to amyloid deposition and
associated calcification may be seen in up to 80-90% of cases
[39].
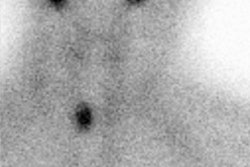
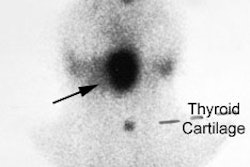
On scintigraphy medullary carcinoma appears as a cold nodule on routine thyroid scanning. I-131 MIBG has been used to image medullary carcinoma, but with only limited success. Pentavalent DMSA [Tc(V)-DMSA] has had limited use in imaging these tumors. It is reported to be more sensitive than MIBG in tumor detection. The agent exhibits characteristics comparable to the orthophosphate ion and this may aid in its localization to medullary carcinoma due to calcification within the tumor. In-111 Octreotide (a somatostatin analog) has also been demonstrated to accumulate within the tumor, but the sensitivity is only about 50% [17] (See also In-111 Octreotide Tumor Imaging).
Because the lesion is actively metabolic, FDG PET imaging can
have a sensitivity up to 96% [17] and FDG appears to be superior
to 18F-DOPA in patients with short calcitonin doubling
times (less than 12 months- likely due to increased tumor
aggressiveness and higher glucose metabolism) [18]. However, other
authors suggest that FDG imaging is more sensitive only in
patients with unstable CEA doubling times [29]. FDG has been
reported to have a low detection rate for metastases in MTC when
the serum calcitonin level is less than 1000 pg/mL, but a
significantly increased detection rate when the level is greater
than 1000 pg/mL [39]. 18F-DOPA has been shown to
detect more tumor foci than conventional imaging and the agent has
been suggested to be more sensitive than FDG for the detection of
MTC in patients with serum calcitonin levels greater than 500 ng/L
and longer calcitonin doubling times [18]. Overall sensitivity for
lesion detection with 18F-DOPA is about 58-70%
[18,19,29]. Premedication with the decarboxylase inhibitor
carbidopa may aid in inhibiting peripheral decaboxylation of the
agent and enhance tumor uptake of DOPA [29].
SSTR types 2 and 5 are frequently expressed in medullary thyroid
cancer and therefore, 68Ga-DOTATE can be used for
imaging [45]. 68Ga-DOTATE has been shown to be
superior to In-111 octreotide SPECT/CT for the detection of
recurrent MTC and distant metastatic disease, but it is still less
sensitive than 18F-DOPA imaging (72% for 18F-DOPA,
vs 33% for 68Ga-DOTATE) [45].
Primary Thyroid Lymphoma
Thyroid lymphoma accounts for less than 5% of thyroid malignancies and is usually of B-cell origin [8]. It is seen more commonly in women than in men [8]. It usually presents as a rapidly enlarging goiter. An increased risk for thyroid lymphoma is seen in patients with chronic lymphocytic thyroiditis [8]. Treatment is XRT and there is about 50% survival at 10 years. Antimicrosomal and antithyroglobulin antibody levels may be elevated in patients with primary thyroid lymphoma. Secondary involvement of the thyroid with lymphoma is seen in about 20% of patients in autopsy series. Thyroid lymphoma does not concentrate radioiodine.
REFERENCES:
(1) Radiol Clin North Am 1993; Price DC. Radioisotopic evaluation of the thyroid and the parathyroids. 31: 991-1015
(2) Endocrinology and Metabolism Clinics of North America 1990; Gorlin JB, Sallan SE. Thyroid cancer in childhood. 19: 649-661
(3) J Nucl Med 1998; Samuel AM, et al. Pulmonary metastases in children and adolescents with well-differentiated thyroid cancer. 39: 1531-1536
(4) Endocrinology and Metabolism Clinics of North America 1990; Hay ID. Papillary thyroid carcinoma. 19: 545-571
(5) J Nucl Med 1993; Bockisch A, et al.Optimized dose planning of radioiodine therapy of benign thyroidal diseases. 34: 1632-38
(6) J Nucl Med Technol 1993; Bender JM, Dworkin HJ. Iodine-131 as an oncology agent. 21: 140-150
(7) Eur J Nucl Med 2001; Petrich T, et al. Outcome after radioiodine therapy in 107 patients with differentiated thyroid carcinoma and initial bone metastases: side-effects and influence of age. 28: 203-208
(8) J Nucl Med 1992; Scott AM, et al. Comparison of technetium-99m-MIBI and thallium-201-chloride uptake in primary thryoid lymphoma. 33: 1396-1398
(9) American Journal of Medicine 1992; Mazzaferri E. Thyroid cancer in thyroid nodules: Finding a needle in the haystack. 93: 359-361
(10) Cancer 2002; Chow SM, et al. Follicular thyroid carcinoma. Prognostic factors and the role of radioiodine. 95: 488-98
(11) J Nucl Med 2003; Lowe VJ, et al. 18F-FDG-PET of patients with Hurthle cell carcinoma. 44: 1402-1406
(12) J Nucl Med 2004; Faggiano A, et al. Age-dependent variation of follicular size and expression of iodine transporters in human thyroid tissue. 45: 232-237
(13) J Nucl Med 2004; Teunissen JJM, et al. Peptide receptor radionuclide therapy for non-radioiodine-avid differentiated thyroid carcinoma. 46: 107S-114S
(14) Radiology 2005; Frates MC, et al. Management of thyroid nodules detected at US: society of radiologists in ultrasound consensus conference statement. 237: 794-800
(15) Thyroid 2006; Cooper DS, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. 16: 1-25
(16) Radiographics 2006; Scarsbrook AF, et al. Multiple endocrine neoplasia: spectrum of radiologic appearances and discussion of a multitechnique imaging approach. 26: 433-451
(17) Radiographics 2007; Intenzo CM, et al. Scintigraphic imaging of body neuroendocrine tumors. 27: 1355-1369
(18) J Nucl Med 2008; Koopmans KP, et al. 18F-dihydroxyphenylalanine PET in patients with biochemical evidence of medullary thyroid cancer: relation to tumor differentiation. 49: 524-531
(19) J Nucl Med 2008; Jager PL, et al. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. 49: 573-586
(20) JAMA 2006; Mazzaferri EL. Managing small thyroid cancers. 295: 2179-2182
(22) Radiology 2008; Johnson NA, Tublin ME. Postoperative surveillance of differentiated thyroid cancer: rationale, techniques, and controversies. 249: 429-444
(23) AJR 2009; Park JS, et al. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. 192: 66-72
(24) AJR 2009; Choi JS, et al. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. 193: 871-878
(25) AJR 2010; Sillery JC, et al. Thyroid follicular carcinoma: sonographic features of 50 cases. 194: 44-54
(26) AJR 2010; Lee S, et al. Medullary thyroid carcinoma: comparison with papillary thyroid carcinoma and application of current sonographic criteria. 194: 1090-1094
(27) AJR 2010; Wong KK, et al. Staging of differentiated thyroid
carcinoma using diagnostic 131I SPECT/CT. 195: 730-736
(28) J Clin Endocrinol Metab 2008; Brown AP, et al. The risk of
second primary malignancies up to three decades after the
treatment of differentiated thyroid cancer. 93: 504-515
(29) J Nucl Med 2011; Kauhanen S, et al. Complementary roles of 18F-DOPA
PET/CT and 18F-FDG PET/CT in medullary thyroid
cancer. 52: 1855-1863
(30) J Nucl Med 2012; Avram AM. Radioiodine scintigraphy with
SPECT/CT: an important diagnostic tool for thyroid cancer staging
and risk stratification. 53: 754-764
(31) J Nucl Med 2012; Salaun PY, et al. Phase II trial of
anticarcinoembryonic antigen pretargeted radioimmunotherapy in
progressive metastatic medullary thyroid carcinoma: biomarker
response and survival improvement. 53: 1185-1192
(32) Radiology 2012; Maruoka Y, et al. Incremental diagnostic
value of SPECT/CT with 131I scintigraphy after
radioiodine therapy in patients with well-differentiated thyroid
carcinoma. 265: 902-909
(33) JAMA 2013; Xing M, et al. Association between BRAF V600E
mutation and mortality in patients with papillary thyroid cancer.
309: 1493-1501
(34) Radiographics 2014; Nachiappan AC, et al. The thyroid:
review of imaging features and biopsy techniques with
radiologic-pathologic correlation. 34: 276-293
(35) J Nucl Med 2014; Avram AM, Shulkin BL. Thyroid cancer in
children. 55: 705-707
(36) J Nucl Med 2014; Mihailovic J, et al. Recurrent disease in
juvenile differentiated thyroid carcinoma: prognostic factors,
treatments, and outcomes. 55: 710-717
(37) J Nucl Med 2014; Pryma DA, Mandel SJ. Radioiodine therapy
for thyroid cancer in the era of risk stratification and
alternative targeted therapies. 55: 1485-1491
(38) J Nucl Med 2015; Nagarajah J, et al Correlation of BRAFV600E
mutation and glucose metabolism in thyroid cancer patients: an 18F-FDG
PET study. 56: 662-667
(39) Radiographics 2016; Kelil T, et al. Current concepts in the
molecular genetics and management of thyroid cancer: an update for
radiologists. 36: 1478-1493
(40) J Nucl Med 2016; Pattison DA, et al. A new theranostic
paradigm for advanced thyroid cancer. 57: 1493-1494
(41) J Nucl Med 2016; Bodet-Milin C, et al. Immuno-PET using
anticarcinoembryonic antigen bispecific antibody and 68Ga-labeled
peptide in metastatic medullary thyroid carcinoma: clinical
optimization of the pretargeting parameters in a firest-in-human
trial. 57: 1505-1511
(42) J Nucl Med 2017; Yang X, et al. TERT promoter mutation
predicts radioiodine-refractory character in distant metastatic
differentiated thyroid cancer. 58: 258-265
(43) Radiology 2017; Cox VL, et al Cancer
genomics and important mutations: a contemporary guide for body
imagers. 283: 314-340
(44) J Nucl Med 2018; Werner RA, et
al. Predictive value of 18F-FDG PET in patients with
advanced medullary thyroid carcinoma treated with vandetanib. 59:
756-761
(45) AJR 2018; Sanli Y, et al. Neuroendocrine tumor diagnosis and
management: 68Ga-DOTATE PET/CT. 211: 267-277
(46) J Nucl Med 2018; Schmidt M, et al. A matter of controversy:
is radioiodine therapy favorable in differentiated thyroid
carcinoma? 59: 1195-1201
(47) J Nucl Med 2019; Kreissl MC, et al. Current treatment
strategies in metastasized differentiated thyroid cancer. 60: 9-15
(48) J Nucl Med 2020; Liu J, et al. The genetic duet of BRAF
V600E and TERT promotrer mutations robustly predicts loss of
radioiodine avidity in recurrent papillary thyroid cancer. 61:
177-182