Hepatopulmonary Syndrome:
View cases of hepatopulmonary syndrome
Clinical:
Hypoxemia is seen in one-third of decompensated cirrhotic
patients [3]. Hepatopulmonary
syndrome (HPS) is the triad of chronic liver disease, increased
alveolar-arterial oxygen
gradient during normal breathing on room air, and intrapulmonary
arteriovenous shunting (due to
subpleural arteriovenous microshunts that resemble spider angiomas
[4]).
It is now recognized to develop in 15-20% of patients with liver
cirrhosis [5]. Patients present with the gradual onset of
hypoxemia that is commonly more
severe in the upright position (the shortness of breath is
relieved when lying down), cyanosis, clubbing, and orthodeoxia
(fall in arterial saturation when assuming an upright position)
[4,5,7]. The presumed underlying pathophysiology is excessive
vascular production of vasodilators- particularly nitric oxide
[6]. The diagnosis of HPS is based on the triad of chronic liver
disease, increased alveolar-arterial oxygen gradient of > 15 mm
HG (or 20 mmHg in patients over 64 years of age), and evidence of
intrapulmonary right-to-left shunting [7].
Laboratory abnormalities include hypoxemia, and decreased diffusing capacity for CO2. The majority of patients with HPS demonstrate marked improvement in symptoms when given 100% oxygen. HPS is a relative contraindication to liver transplant surgery, however, some patients with HPS may actually improve following transplant.
X-ray:
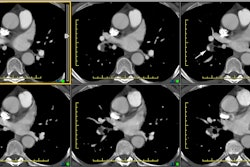
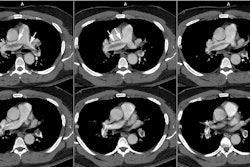
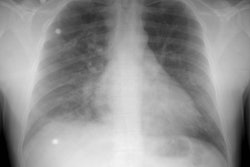
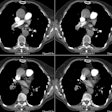
Chest radiograph abnormalities are detected in 46-100% of patients with HPS [3]. Medium sized (1.5 to 3 mm), bilateral, basilar, nodular or reticulonodular opacities, with normal lung volumes, are characteristic of HPS and represent dilated subpleural lung vessels.
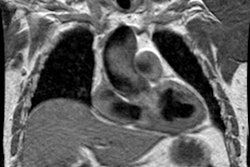
On HRCT dilated subpleural vessels do not tapper normally and are seen to extend to the pleural surface and have an abnormally large number of visible terminal branches. These branches may resemble irregular linear opacities of fibrosis, however, HPS can be distinguished from pulmonary fibrosis by the absence of ground glass opacities, septal lines, architectural distortion, and traction bronchiectasis. Focal nodular dilatation of subpleural vessels can also be seen due to the presence of individual arteriovenous malformations [5]. On pulmonary arteriography dilated pulmonary vessels and subpleural telangiectasias are identified. Although uncommon, macroscopic arteriovenous malformations are a treatable cause of hypoxemia in patients with HPS and arteriography is recommended to exclude AVM's in all patients with abnormal responses to 100% inspired oxygen.
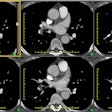
Tc-MAA imaging will demonstrate heterogeneous pulmonary perfusion
and the presence of
right to left shunting with activity outside the lungs in the
brain, liver, and spleen [5]. Regions of interest are drawn around
the brain (lateral views) and lungs (anterior and posterior views)
and radiotracer counts are recorded [7]. The pulmonary shunt
percent is calculated by applying the geometric mean of the brain
and lung counts [7]:
(GM Brain)/(GM Brain + GM lung) x 100 (normal < 6%)
Scintigraphy can also be used to quantify the shunt, but it cannot distinguish between intracardiac and intrapulmonary shunting (best diagnosed by echocardiography with saline microbubble injection).
REFERENCES:
(2) Radiology 1999; Lee, KN, et al. Hypoxemia and liver cirrhosis (hepatopulmonary syndrome) in eight patients: Comparison of the central and peripheral pulmonary vasculature. 211: 549-553
(3) Radiographics 2000; Meyer CA, et al. Diseases of the hepatopulmonary axis. 20: 687-698
(4) Radiographics 2002; Engelke C, et al. High-resolution CT and CT angiography of peripheral pulmonary disorders. 22: 739-764
(5) Radiographics 2009; Kim YK, et al. Thoracic complications of liver cirrhosis: radiologic findings. 29: 825-837
(6) Radiographics 2010; Grosse C, Grosse A. CT findings in
diseases associated with pulmonary hypertension: a current review.
30: 1753-1777
(7) J Nucl Cardiol 2015; Surasi DS, et al. Lung perfusion imaging
in hepatopulmonary syndrome using 99mTc
macroaggregated albumin. 22: 586-588