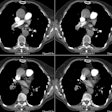
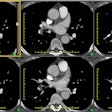
Intramural Hematoma:
View cases of intramural hematoma
Clinical:
An intramural hematoma (IMH) is felt to represent a dissection
of the aortic wall without intimal
rupture or tear. Current opinion is that IMH is a variant or
precursor of aortic dissection [16]. The etiology is not known,
but may be related to: 1- weakening of the aortic wall media
with spontaneous rupture of the aortic vasa
vasorum which leads to subintimal bleeding (bleeding into the
arterial media); 2- intimal
fracture of an atherosclerotic plaque with a microintimal tear;
or 3- intramural propagation of hemorrhage adjacent to a
penetrating atherosclerotic ulcer (the ulcer may or may not be
identified at CT) [2,3,4,11,17].
Intramural hematomas associated with ulcerations are most
commonly observed in the descending thoracic aorta. Intramural
hematoma can be a precursor to frank aortic dissection,
especially when the lesion is found in the ascending aorta [2,3].
Patients with IMH are typically elderly (60-80 years of age
[18]) with a history of hypertension [7] and often have
associated atherosclerotic disease or penetrating
atherosclerotic ulcers. Patients present with symptoms similar
to an aortic dissection with chest pain (50-74%) or back pain
(44-84%) [7], however, patients may be asymptomatic. Acute IMH
accounts for 5-15% of all cases of acute aortic syndrome [16].
IMH is classified according to the Stanford classification system- type A involves the ascending aorta (with or without involvementof the descending aorta) and type B invovles the descending aorta [15,17]. Type B IMH appears to be more common and accounts for 60% of cases [15]. Other authors suggest the lesion occurs with roughly equal frequency in the ascending and proximal descending aorta, and about 8% of cases involve the aortic arch [4].
Ulcer-like projections (also referred to as intimal erosions
[15]) can occur in IMH and appear as a localized contrast filled
pouch (typically larger than 3mm) with an obvious communication
with the true lumen that is not seen on the initial exam
(although other authors suggest that ULPs can be identified when
an IMH is initially diagnosed [18]) [13,17]. Ulcer-like
projections can develop within the lesion in about one-third of
patients- commonly within 1 to 4 months, but the complication
can be delayed [12]. Ulcer-like projections represent a new site
of intimal disruption and carry a
poor prognosis, particularly when located in the ascending aorta
or aortic arch, and frequently progress to dissection, aneurysm,
or rupture [13,17]. A larger ulcer-like projection and depth
correlate with a higher rate of complications (see below) [17].
Another recently described finding that can also be seen in association with IMH is an intramural blood pool (IMBP) or focal contrast enhancement or aortic branch artery pseudoaneurysm [13,14,17,18]. These lesions appear as a small focal island-like contrast collection within the IMH with no obvious connection (or a very tiny orifice- < 2mm) to the true lumen [13,14,17]. An intramural blood pool is more likely to occur in the descending aorta and it is sometimes referred to as an aortic branch tear or an aortic branch artery pseudoaneurysm because there is often a visible connection with an intercostal, lumbar, or brachial artery that pass from the aortic lumen and are disrupted by the IMH (up to 82% of IBP's show either a tiny lumenal orifice or a connection with an intercostal or lumbar artery) [13,17,18]. The lesion typically appears in IMH with a thickness of greater than 10 mm [13,18]. The presence of an IMBP is not necessarily associated with a poor prognosis (no increased risk for IMH progression, need for surgery, or mortality) and more than half will demonstrate resorption on follow-up CT imaging [13]. IMBPs resolve in 44-57% of cases, decrease in 29-33% of cases, and remain stable in 3% [18]. Larger IBP's and those with intercostal or lumbar artery communications have a higher risk of incomplete resorption and may grow over time, necessitating endovascular embolization [13,17]. Other authors have found that the presence of an IMBP is associated with an increased risk for IMH progression [14].
Penetrating aortic ulcers (PAUs) involve ulceration of an
aoritc plaque through the internal elastic lamina into the
media, with secondary variable amounts of medial hematoma [17].
PAUs, unlike ulcer-like projections, protude from the contrast
opacified lumen into the thrombus-containing aortic wall,
enhance to the same degreee as the aortic lumen, typically have
a focal irregular intima, are
associated atherosclerotic plaque, and are usually noted on the
initial scan [13,15]. Atheromatous ulcers have a similar
appearance, but are confined to the intimal layer [16]. PAUs
typically occur in an elderly individual with atherosclerosis
and over 90% are located in the descending thoracic aorta [16].
The incidence of PAU in patients presenting with an acute aortic
syndrome is between 2-8% [16].
In patients with IMH, ulcer-like projections and penetrating
aortic ulcers are associated with an increased risk for
complications/progressive disease course including aneurysm
formation, dissection, rupture, need for surgery, and mortality
[15,16,17]. The greater the diameter and depth of the ulcer
(diameter 10-20mm or depth 5-10mm) increases the risk for
complications [15,17]. The presence of an ulcer-like projection
or penetrating ulcer in the ascending aorta are also associated
with a greater risk for aneurysm formation [15]. The incidence
of complications in patients with PAUs can be as high as 70%
[16].
Progression to frank dissection are more likely to occur for
type A hematomas involving the ascending aorta [11,15]. Type A
IMH also results in increased risk for pericardial or pleural
effusion, aneurysm formation, and death [17]. Aortic diameter
predicts adverse events independent of other potential risk
factors and smaller maximum aortic diameter predicts resolution
of the IMH [15]. An increased risk for adverse events (aneurysm,
dissection, need for surgery, rupture, and death) is also
associated with an underlying aortic aneurysm with an aortic
diameter of greater than 48-55mm (ascending IMH) and greater
than 41mm (descending IMH) [7,10,11,14,15]. Progression to
aneurysm or dissection is also associated with greater thickness
IMH (approximately 11-16mm) [9,15]. Conversely, a thickness of
< 10-11mm predicts a lower risk of complications within 30
days and a greater chance of IMH resolution [15].
On follow-up, lesions that progress to aortic dilatation are
more likely to develop ulcer-like projections and are usually
located within the ascending aorta [8].
Prognosis of IMH is variable, but available data suggests that
the clinical course of IMH mimics acute aortic dissection with
similar mortality rates [7]. Because of weakness of the aortic
wall in the segment with the intramural hematoma, aortic
remodeling of the diseased area can occur [14]. Between 10-33%
may regress over months to years [9,16], progression to classic
aortic dissection is seen in 28-47% of cases [16], and between
33-56% of cases progress to form an aortic aneurysm or pseudoaneurysm [7,9]. The risk of aortic
rupture in IMH is 20-45% [16]. The mortality from IMH in the
first 3 months of evolution is high [9].
Prognosis also depends on the site of the IMH. Studies have also shown a high rate of complications and mortality for hematomas that involve the ascending aorta [11]. The mortality from proximal aortic intramural hematoma is higher than that of distal IMH (34-55% versus 14-19%) and most deaths tend to occur within the first 24 to 72 hours after hospital admission [7,9]. Predictors of early mortality or adverse outcome include a maximum aortic diameter of greater than 5 cm, an IMH thickness of greater than 11mm, and ascending aortic involvement [9].
Treatment of the lesion is similar to aortic dissection. Observation and medical therapy (antihypertensives) are used for patients with lesions in the descending aorta (type B IMH) [7]. For lesions involving the ascending aorta (type A), those with recurrent hematoma, or those with increasing aneurysm size, surgery or stent graft placement is preferred [7,9,11,16]. The reported mortality for medically treated type A IMH is approximately 40% [18]. Because of the incidence of late complications and anuerysm formation patients with IMH require periodic aortic imaging [7].
X-ray:
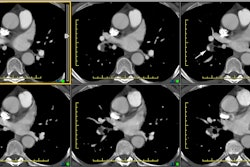
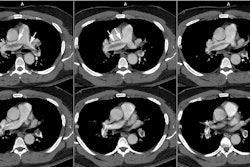
Computed Tomography: On non-contrast exams, the intramural hematoma produces a crescent-shaped area of mural thickening which frequently has increased attenuation reflecting acute hemorrhage (diameter > 7mm; attenuation 60-70 HU [11]). The lesion will also displace intimal calcifications- the displaced calcifications usually appear semicircular or cirular and curvilinear [11]. The surrounding mediastinal fat often demonstrates increased attenuation as well. Intense enhancement of the aortic wall external to the hematoma can be seen and may reflect adventitial inflammation. It is important to document the maximal aortic diameter, the maximal axial thickness of the hematoma, and the minimum and maximum transverse diameters of the aortic lumen at the level of the hematoma [11]. On unenhanced imaging performed within as little as 1 week after the onset of symptoms the hematoma may show an attenuation identical to that of intramural blood [11].
On follow-up exams, the hematoma typically decreases in size and may resolve- especially during the first year following development of the hematoma [10]. After one year, however, the aorta may develop a fusiform aneurysm or a true dissection [5]. This may be the result of structural weakness of the aortic wall as a result of the hematoma and the continuous mechanical stress from pulsating blood flow [10]. A penetrating aortic ulcer can be seen in association with an intramural hematoma at presentation, or develop during the follow-up period- especially in the region of the distal aortic arch [5]. In one study, approximately one-third pf patients developed an ulcer-like projection within the first 3 months of followup [11]. The penetrating ulcers can progress to saccular aneurysm (pesudoaneurysm) formation or may resolve [5,11].
An intramural hematoma can be difficult to distinguish from
atherosclerotic mural thickening. A key point to remember for
detecting ascending aortic intramural hematomas is that
atherosclerotic plaques are typically not present in the
ascending aorta. Intramural hematomas also extend smoothly in a
longitudinal direction, while plaque typically has a more
irregular surface. Differentiation from a thrombosed
false lumen of an aortic dissection may be aided by the fact
that dissections tends to spiral longitudinally around the
aorta, while intramural hematomas maintain a constant
circumferential relationship.
The CT report should include: the Stanford classification;
maximum aortic diameter; maximum hematoma thickness (measured
perpendicular to the longitudinal axis in the axial plane);
presence or absence of focal contrast enhancement (intramural
blood pool or ulcer-like projection); ulcer-like projection
diameter and depth; and presence or absence of pleural effusion,
pericardial effusion, or periaortic hematoma [17].
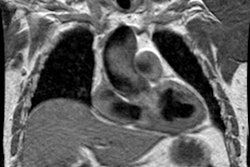
Magnetic Resonance Imaging: On T1 MR the intramural hematoma appears as a crescentic to circumferential area of thickened aortic wall which may be isointense (within 7 days of onset of symptoms) or hyperintense (after 7 days) relative to skeletal muscle depending on the age of the lesion (as oxyhemoglobin is converted to methemoglobin). On gradient-echo (white blood) images, an acute intramural hematoma (age < 7 days) shows T2 hyperintensity, while subacute or chronic hematoma (over 7 days) has intermediate T2 signal intensity [11]. There is no evidence of an intimal flap or penetrating aortic ulcer. Cine gradient images demonstrate no change in signal intensity confirming the lack of flow.
REFERENCES:
(1) Magn Reson Imaging Clin N Am 1996; 4(2): 217-235
(2) Radiology 1997; Bluemke DA. Definitive diagnosis of intramural hematoma of the thoracic aorta with MR imaging. 204: 319-321 (No abstract available)
(3) Radiology 1997; 204: 349-355
(4) J Thorac Imag 1997; 12: 128-149
(5) J Comput Assist Tomogr 1997; Sueyoshi E, et al. Fate of intramural hematoma of the aorta: CT evaluation. 21 (6): 931-938
(6) Radiographics 1999; Sebastia C, et al. Aortic dissection: Diagnosis and follow-up with helical CT. 19: 45-60
(7) Chest 2001; Neilander S, et al. Aortic intramural hematoma. An increasingly recognized and potentially fatal entity. 120: 1340-1346
(8) Radiology 2002; Sueyoshi E, et al. New development of an ulcerlike projection in aortic intramural hematoma: CT evaluation. 224: 536-541
(9) Radiol Clin N Am 2005; Chiles C, Carr JJ. Vascular diseases of the thorax: evaluation with multidetector CT. 43: 543-569
(10) AJR 2006; Sueyoshi E, et al. CT analysis of the growth rate of aortic diameter affected by acute type B intramural hematoma. 186: S414-420
(11) Radiographics 2009; Chao CP, et al. Natural hsitory and CT appearance of aortic intramural hematoma. 29: 791-804
(12) AJR 2009; Bosma MS, et al. Ulcerlike projections developing in noncommunicating aortic dissections: CT findings and natural history. 193: 895-905
(13) Radiology 2011; Wu MT, et al. Intramural blood pools accompanying aortic intramural hematoma: CT appearance and natural course. 258: 705-713
(14) Radiology 2011; Park GM, et al. Distal aortic intramural
hematoma: clinical importance of focal contrast enhancement on
CT images. 259: 100-108
(15) J Cardiovasc Comput Tomogr 2013; Kruse MJ, et al. Aortic
intramural hematoma: review of high-risk imaging features. 7:
267-272
(16) AJR 2014; Maddu KK, et al. Nontraumatic acute aortic
emergencies: Part I, acute aortic syndrome. 202: 656-665
(17) Radiographics 2016; Gutschow SE, et al. Emerging concepts
in intramural hematoma imaging. 36: 660-674
(18) Radiographics 2021; Ko JP, et al. Chest CT angiography for acute aortic pathologic conditions: pearls and pitfalls. 41: 399-424