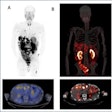
GE Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for CoreScan, an application that quantifies visceral adipose tissue (VAT) during body composition analysis.
The ability to quantify visceral "belly" fat is now available on GE Lunar's iDXA body composition system, according to the vendor.