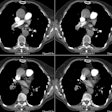
Aortic Dissection:
View cases of aortic dissection
Clinical:
Aortic dissection results form an intimal tear (hematoma) within
the aorta that creates a false passage through the aortic media.
The right lateral wall of the ascending aorta and the proximal
segment of the descending thoracic aorta are the sites of maximal
hydralic stress and are the most common sites of an entrance tear
in the intima [16]. The false lumen lies within the outer half of
the media, so that the outer wall of the false lumen is very thin-
usually about one-quarter as thick as the original aortic wall
[9]. Pressures within the false lumen are equal to or greater than
those in the true lumen [13]. If the dissection ruptures back
through the endothelium at some distance from the origin, a false
channel with flowing blood will ensue.
Atherosclerotic disease and hypertension (occurs in 60-90% of
cases [13]) are the most common causes. Other etiologies include:
Connective tissue disorders- cystic medial necrosis which is seen
in Marfan's (Marfans syndrome is reported in about 5% of patients
with dissection [13]) and Ehler's-Danlos, as well as Loeys-Dietz
syndrome; trauma/iatrogenic causes such as following arterial
catheterization (inflammatory vasculitides such as Takayasu
arteritis and giant cell arteritis can increase the risk of aortic
dissection with instrumentation) [20] or prior aortic surgery;
aortic stenosis/coarctation; aortic aneurysm [13]; infection
(syphilis) and other causes of aortitis; and pregnancy [8].
Cocaine use has also been associated with aortic dissection in
otherwise healthy, normotensive patients [8,13]. Inflammatory
vasculitides such as t
The most common sites for dissections to originate are the tubular portion of ascending aorta and the junction of arch and descending aorta. Although most dissections originate in the proximal ascending portion of the vessel, these are usually associated with patient mortality (tears which involve the ascending aorta may result in hemopericardium and this is not good for one's health), and are therefore not seen as often clinically as those which originate just beyond the origin of the left subclavian artery. The dissection can be classified in one of 2 manners- either by the DeBakey method or the Stanford method.
DeBakey Classification:
I: Ascending and Descending aortic dissection: Most common
(70%)
II: Ascending: Least common
III: Descending
Stanford Classification:
A: Ascending - most common- account for 60-75% of cases,
originates proximal to the inominant artery [13,17].
B: Descending- involves the descending aorta distal to the left
subclavian artery (30-40% of cases) [13,17].
One drawback of the above classification systems is that neither
addresses dissections that originate in the aortic arch which can
occur in about 15% of cases [17]. From a surgical perspective,
arch dissections are viewed as a variant of a type-B dissection
[17]. Current surgical recommendations are to delay surgical
intervention for arch dissections and provide medical treatment
unless there is evidence of end-organ ischemia or patient
instability [17].
Patients may present with the classic history of acute-onset
central chest pain that radiates to the back [13]. However, it has
been reported that up to 20% of patients may present with syncope
without a history of typical pain [13]. Aortic regurgitation can
produce a diastolic murmur and can be found in 40-50% of patients
with proximal dissections [13]. Dissections of less than 14 days
since the onset of symptoms are considered acute, where as those
beyond this interval are considered chronic [14]. Isolated
descending dissections are treated medically with antihypertensive
control, unless there is proximal extension, hemodynamic
instability, a diameter of greater than 6 cm, distal embolization,
or ischemia to major organs that would necessitate surgical
intervention [13].
Complications associated with ascending dissections include proximal extension into the aortic valve resulting in aortic insufficiency, intrapericardial rupture and tamponade, carotid artery dissection with stroke, and coronary artery dissection or origin from the false lumen with subsequent infarction [12].
Aortic dissections are the most common life-threatening disorder
of the aorta with a mortality rate as high as 1% per hour [18].
Early surgical intervention is indicated for all patients with
type A aortic dissection [10]. If untreated, type A dissections
are associated with a mortality rate of over 50% within 48 hours
[13]. Treatment for ascending dissections is surgical using a
continuous suture-graft technique between the coronary ostia and
the proximal aortic arch [10]. Post-op complications include
anastomotic leak or frank rupture, perigraft infection, recurrent
dissection, anastomotic stenosis, and pseudoaneurysm formation due
to partial dehiscence along the suture line [10]. A periprosthetic
hematoma appears as a asymmetric thickening of the lumen near the
graft exceeding 10 mm (concentric thickening of less than 10mm can
be a normal post-operative finding) [10]. A periprosthetic
hematoma of greater than 15 mm following surgery places the
patient at an increased risk for pseudoaneurysm formation [10].
Three risk factors are associated with an increased risk for
mortality with type B dissection- hypotension or shock, lack of
chest or back pain at presentation, and branch vessel involvement
[9]. At follow-up, favorable remodeling is suggested by a decrease
in the false lumen of more than 10% or an increase in the true
lumen of more than 10% in diameter (with a stable or smaller
overall aortic diameter) [19]. Type B dissections can be
complicated by the development of an aortic aneurysm (20-50% of
cases) or a distinct enlargement of the false lumen which produces
a narrowing of the true lumen [5]. Imaging risk factors predictive
of risk for enlargement of the descending aorta are a large
primary intimal tear (>10mm); a descending aortic diameter of
more than 35mm; a false lumen diameter of more than 22mm in the
proximal descending aorta; a false lumen diameter of more than
2/3'ds of the total descending aortic diameter; a partially
thrombosed distal false lumen, as opposed to a patent or
completely thrombosed false lumen; and helical flow in the false
lumen [19].
Endovascular stent grafting has been used with success to treat
type B dissections [5,7,11]. In these cases, it is important to
cover the entry tear which will provide promote thrombosis of the
false lumen [11]. Common indications for stenting include
resistent hypertension, refractory pain, aortic diameter > 4 cm
in the acute to subacute setting with a persistent false lumen;
aortic diameter > 5.5 cm in chronic dissection, aortic
expansion of > 10 mm in 3 months in subacute dissection
or > 5 mm in 6 months in chronic dissection, false lumen
expansion > 1 cm within one year, branch vessel obstruction or
compromise, impending rupture, or clinical malperfusion with
imaging of dynamic collapse of the true lumen [19].
Limited intimal tear is a partial thickness tear that occurs in
patients with abnormal aortic media and appears as a
pseudoaneurysm [20]. It demonstrates no significant separation of
the aortic layers or hematoma, and there is no associated false
lumen or dissection flap [20]. There is a linear or stellate tear
of the intima, and to a variable degree of the media, which
exposes the residual media and adventitia, causing an outward
bowing of the the affected aortic wall [20.
X-ray:
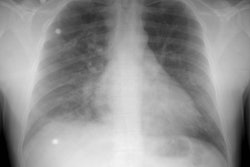
CXR:
CXR findings may be normal in 10-40% of patients [13]. Plain film findings which suggest dissection include aortic widening with obliteration of the aortic knob (seen in 45% of type A and 49% of type B dissections [9]), widening of the superior mediastinum (seen in 62% of type A and 56% of type B dissections [9]), and wall calcifications which are displaced more than 6 mm from the outer aspect of the aorta. A pleural effusion can be seen in up to 19% of patients with type A dissection [9].
Transesophageal US:
TEUS can be performed at the patient's bedside and has a reported
sensitivity of about 94-100% for the detection of dissection, but
the distal part of the ascending aorta and the branches of the
aortic arch may not be adequately evaluated with TEUS [13]. TUES
is also able to identify aortic valvular insufficiency, which in
the setting of acute aortic dissection indicates the need for
emergent valve replacement in addition to aortic repair.
Trans-esophageal echo is probably the procedure of choice for the
detection of aortic dissection in hemodynamically unstable
patients. False positive results can occur due to reverberation
artifacts and false negative exams can occur due to a poor
acoustic window or large body habitus [18].
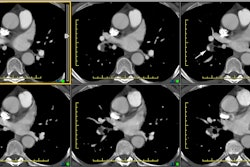
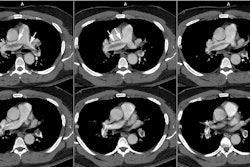
Computed tomography/ Helical CT:
A non-contrast localization scan should be obtained before contrast administration to detect intramural hematoma. Scanning should be performed from the thoracic inlet to the top of the L5 vertebral body [4].
Contrast injection into the right antecubital vein is preferred as a left antecubital injection results in opacification of the left brachiocephalic vein. Concentrated contrast in the left brachiocephalic vein can create perivenous streak artifact that may obscure visualization of the origins of the great vessels from the aortic arch. Pulsation artifact may be seen in the ascending aorta and can simulate a dissection. Segmenting the reconstruction (partial reconstruction of a less than 360 degree scan) helps eliminate this pitfall [3]. ECG gating can also be performed to minimize cardiac motion artifacts and allows clear delineation of the aortic root and coronary artery involvement (however, unless performed with prospective gating, the exam does have a greater radiation dose) [13]. Overlapping reconstructions will improve quality of multiplanar reformat images [4]. CT has an accuracy rate of about 92% for the diagnosis of dissection [4]. Limitations of CT relate to its inability to delineate aortic insufficiency or coronary artery involvement.
The diagnostic hallmark for dissection is the presence of an
intimal flap which divides two contrast columns with delayed
opacification of the false lumen. Separation of calcifications
from the outer aortic contour (must be careful not to misinterpret
volume averaging artifacts) may be seen- especially on the
non-contrast exam. In most cases the identity of the true lumen
can be determined by its continuity with an undissected portion of
the aorta [6]. However, in some patients this may be difficult to
appreciate [6]. The false lumen is usually anterior and to the
right in the ascending aorta, superior and slightly posterior
along the arch, and posterior and to the left in the descending
aorta. Below the diaphragm, it can spiral either anteriorly to
involve the mesenteric vessels, or posteriorly [13]. The false
lumen of the dissection will usually compresses the true lumen. A
multi-barreled appearance (with multiple false lumens) can occur
in 5-9% of patients and is associated with a worse prognosis [15].
Other features which can be useful for identification of the true and false lumen include: (See: CT Features to Distinguish the True from False Channel)
1- The beak sign: Small "beaks" of contrast or thrombus can usually be identified at the lateral margins of the false lumen [6].
2- Cobwebs: Cobwebs are ribbons of media which are incompletely sheared off by the dissection and are located exclusively in the false lumen [6,9] and the sign is highly specific in the identification nof the aortic false lumen [16]. Cobwebs can be found in up to 9% of acute dissections and 17% of chronic dissections [6]. The strands can range in size from barely noticeable to several millimeters thick [9].
3- Outer wall calcification: Although outer wall calcifications may be seen in the false lumen of a chronic dissection, they are generally not seen in the outer wall of the false lumen of an acute dissection (ie: outer wall calcifications imply the true lumen) [6].
4- Eccentric calcifications along the dissection flap: Calcifications along the dissection flap are usually located along the true lumen side of the flap [6].
5- Intralumenal thrombus: Intralumenal thrombus is more commonly seen in the false lumen of both acute and chronic dissections, although in patients with aortic aneurysms thrombus may be present in the true lumen as well [6].
6- Larger lumen: The larger lumen is usually the false lumen [6,9].
7- Wrap around: In patients with involvement of the aortic arch, it is common for one lumen to appear to wrap around the other lumen (sometimes giving the appearance of three lumens). In such cases, it is the false lumen that wraps around the true lumen [6].
8- Wind-sock appearance- a circumferential intimal flap occurs due to dissection of the entire intima [13]. A narrow true lumen with a fusiform shape may be seen and an intimointimal intussusception can occur [13].
9- Mercedes-Benz sign- a three-channel dissection in which a secondary dissection occurs within one of the channels [13].
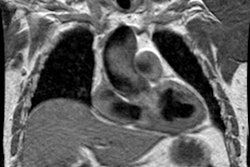
Magnetic resonance imaging:
MRI is highly sensitive and specific in the detection of aortic dissection. It can be performed without the use of intravenous contrast, however, the exam may be difficult to perform in an acutely ill patient. Usually, the false lumen will be anterior and rightward of the true lumen in the ascending aorta, superior to the true lumen in the transverse arch, and posterior and to the left of the true lumen in the descending thoracic aorta. Cine gradient images offer the advantage of being able to detect retrograde flow within the false lumen that may possibly result in retrograde extension of a type B dissection. On T1 images, slow flow may be seen as intermediate signal intensity within the false lumen which will mimic thrombus- cine images may be useful to differentiate the two. On cine images, thrombus will remain of intermediate signal intensity, while slow flow will demonstrate cyclical enhancement. Calcifications are not identified on the MRI exam and a type B dissection with a clotted false lumen may be difficult to distinguish from thrombus within an aortic aneurysm. Signs that suggest a clotted false lumen include compression or eccentric patent lumen (as opposed to a concentric lumen associated with thrombus) and extensive thrombus with associated wall thickening extending over a length of greater than 7 cm.
Post-op CT/MRI findings include uniform, concentric perigraft thickening and a residual intimal flap distal to the graft can be seen in up to 50% of cases. Both channels distal to the graft are usually preserved to ensure continued flow to organs now dependent upon the false lumen. Perigraft flow may represent a protected pseudoaneurysm contained by the perigraft wrap.
REFERENCES:
(1) Magn Reson Imaging Clin N Am 1996; 4(2): 217-235
(2) J Thorac Image 1997; 12: 128-149
(3) Radiology 1997; 205: 153-157
(4) Radiology 1999; Novelline RA, et al. Helical CT in emergency radiology. 213: 321-339
(5) Radiology 2000; Czermak BV, et al. Treatment of Stanford type B aortic dissection with stent-grafts: Preliminary results. 217: 544-550
(6) AJR 2001; LePage MA, et al. Aortic dissection: CT features that distinguish true lumen from false lumen. 177: 207-211
(7) Radiology 2001; Kang SG, et al. Aortic dissection: Percutaneous management with a separating stent-graft - Preliminary results. 220: 533-539
(8) Radiographics 2003; Castaner E, et al. CT in nontraumatic acute thoracic aortic disease: typical and atypical features and complications. 23: S93-S110
(9) Radiol Clin N Am 2005; Chiles C, Carr JJ. Vascular diseases of the thorax: evaluation with multidetector CT. 43: 543-569
(10) Radiographics 2006; Garcia A, et al. MR angiographic evaluation of complications in surgically treated type A aortic dissection. 26: 981-992
(11) AJR 2009; Hoang JK, et al. MDCT angiography of thoracic aorta endovascular stent-grafts: pearls and pitfalls. 192: 515-524
(12) AJR 2009; Litmanovich D, et al. CT and MRI in diseases of the aorta. 193: 928-940
(13) Radiographics 2010; McMahon MA, Squirrell CA. Multidetector
CT of aortic dissection: a pictoral review. 30: 445-460
(14) AJR 2012; Maldjian PD, Partyka L. Intimal tears in throacic
aortic dissection: appearance on MDCT with virtual angioscopy.
955-961
(15) Radiology 2013; Sueyoshi E, et al. Comparision of outcome in
aortic dissection with single false lumen versus multiple false
lumens: CT assessment. 267: 368-375
(16) AJR 2014; Maddu KK, et al. Nontraumatic acute aortic
emergencies: Part I, acute aortic syndrome. 202: 656-665
(17) Radiology 2014; Lempel JK, et al. Aortic arch dissection: a
controversy of classification. 271: 848-855
(18) Radiographics 2017; Malik SB, et al. Transthoracic
echocardiography: pitfalls and limitations as delineated at
cardiac CT and MR imaging. 37: 383-406
(19) Radiographics 2018; Saremi F, et al. Image predictors of
treatment outcome after thoracic aortic dissection repair. 38:
1949-1972
(20) Radiographics 2021; Murillo H, et al. Aortic dissection and
other acute aortic syndromes: diagnostic imaging findings from
acute to chronic longitudinal progression. 41: 425-446