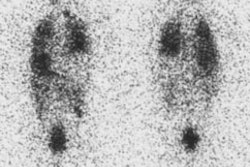
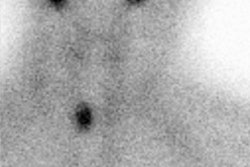
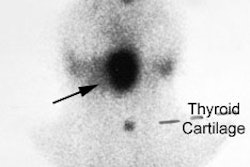
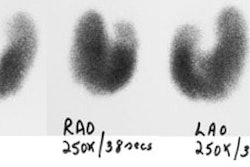
Guidelines for outpatient I-131 radioablation therapy:
From Associates in Medical Physics, LLC
On May 29, 1997, the NRC amended their regulations regarding the
criteria for the
release of individuals containing radioactive substances (10CFR35.75).
Previously, the criteria for release was when either (1) the dose
rate
from the patient
was <5 mrems/hr at 1 meter, or (2) the retained activity in the
patient was <30 mCi.
This was an activity-based release limit and it was deemed faulty
because different
radionuclides with the same activity will give different doses
depending upon the
effective half-life of the radionuclide in the body, as well as
other
factors that vary
for different materials.
The NRC has three criteria for the release of patients from the
hospital following I131 treatment [3]:
1. No individual member of the public is likely to receive more
than
5 mSv (500 mrem) total effective dose equivalent from the patient
in
any year (1 mSv if the individual is pregnant or is 8
years old or less [1]). Verbal and written instructions describing
methods to limit the dose to others must be given to the patient
if an
individual member of the public is likely to receive a dose
exceeding 1
mSv (100 mrem) from the patient, and if the administered dose is
more
than 0.26 Gbq (7 mCi) [3].
2. When the survey meter reading is less than 0.07 mSv/h (7.0
mrem/h) at one meter
3. When the administered activity is 1.22 GBq (33mCi) or less
The patient should be made aware that in addition to radiation
emitted
directly from them, radioiodine is also excreted in body fluids
such as
sweat, saliva,
urine, and feces [2]. After discharge patients should follow
radiation
safety
precautions to limit radiation to
other individuals these include avoiding prolonged/close contact
with other people (especially infants, children, and pregnant
women);
mouth to mouth contact including sharing eating utensils should be
avoided. The patient must sleep alone and abstain from intercourse
for
approximately 1 week after therapy [3]. However, other authors
suggest that less stringent requirements would still result in
acceptable levels of exposure to other individuals [4]. For doses
between 100-200 mCi, sleeping separate for 1 day (or 3 days of
sleeping apart for pregnant women and children) would still meet
recommended exposure guidelines [4]. Plates and utencils used by
the
patient should be washed separately. Clothes may be washed
separately, but this is not required [3]. The patient may use
disposable dishes and utensils- however, when
disposed, these may trigger sensitive waste facility alarms [3].
The
patient should flush the toilet 2 times after
each use and male patients should sit to urinate [3]. The
physician
should be reasonably certain that these instructions
can and will be followed. A record documenting justification of
the
assigned occupancy
factors and the effective half-lives used in the exposure
calculations
must be maintained
for 3 years after the date of patient release [1].
This new regulation also discusses the dose to the breast-feeding
infant or child. If that
dose is likely to exceed 0.1 rem (1 mSv) - assuming no
interruption of
breast-feeding -
instructions are required to address guidance on the interruption
or
discontinuation of
breast-feeding and on the consequences if the patient refuses to
follow
the guidance. This
is of particular concern with I-131 for both diagnostic and
therapeutic
administrations
(e.g.:more than 0.4 uCi I-131). If the mother does not cease
breast-feeding for this
infant or child, particularly in the case of therapeutic
administrations, the internal
dose to the breast-feeding infant could be large enough to cause
hypothyroidism or total
ablation of the infant's thyroid gland. If either one of these
conditions went undetected
and untreated, severe mental retardation could result.
If the mother agrees to cease or interrupt breast-feeding, the new
regulation indicates
the time periods that breast-feeding should be interrupted for
significant radionuclides
(e.g.:>30 mCi Tc-99m pertechnetate - interrupt breast feeding
for 6
hrs.). In
the case of I-131 NaI, complete cessation for this child is
recommended.
The last part of this new regulation (35.75(d) indicates that,
even
though instructions
must be given if the dose exceeds 0.1 rem (1mSv), records of those
instructions must be
maintained only if the dose to the infant or child would exceed
0.5 rem
(5 mSv) -
(e.g.:>200 uCi Ga-67 Citrate). These records must be maintained
for
a period of 3 years
after the release date of the patient.
[Code of Federal Regulations]
[Title 10, Volume 1, Parts 1 to 50]
[Revised as of January 1, 2000]
From the U.S. Government Printing Office via GPO Access
[CITE: 10CFR35.75]
[Page 549]
TITLE
10--ENERGY
COMMISSION
PART 35--MEDICAL USE OF BYPRODUCT MATERIAL--Table of Contents
Subpart
C--General
Technical Requirements
Sec. 35.75 Release of individuals
containing
radiopharmaceuticals
or permanent implants.
(a) The licensee may authorize the release from
its
control of any
individual who has been administered radiopharmaceuticals or
permanent
implants containing radioactive material if the total effective
dose
equivalent to any other individual from exposure to the released
individual is not likely to exceed 5 millisieverts (0.5 rem).\1\
---------------------------------------------------------------------------
\1\Regulatory Guide 8.39, ``Release of Patients
Administered
Radioactive Materials,'' describes methods for calculating doses
to
other individuals and contains tables of activities not likely to
cause
doses exceeding 5 millisieverts (0.5 rem).
---------------------------------------------------------------------------
(b) The licensee shall provide the released
individual with
instructions, including written instructions, on actions
recommended to
maintain doses to other individuals as low as is reasonably
achievable
if the total effective dose equivalent to any other individual is
likely
to exceed 1 millisievert (0.1 rem). If the dose to a
breast-feeding
infant or child could exceed 1 millisievert (0.1 rem) assuming
there
were no interruption of breast-feeding, the instructions shall
also
include:
(1) Guidance on the interruption or
discontinuation
of breast-
feeding and
(2) Information on the consequences of failure
to
follow the
guidance.
(c) The licensee shall maintain a record of the
basis for
authorizing the release of an individual, for 3 years after the
date of
release, if the total effective dose equivalent is calculated by:
(1) Using the retained activity rather than the
activity
administered,
(2) Using an occupancy factor less than 0.25 at
1
meter,
(3) Using the biological or effective
half-life, or
(4) Considering the shielding by tissue.
(d) The licensee shall maintain a record, for 3
years after the date
of release, that instructions were provided to a breast-feeding
woman
if
the radiation dose to the infant or child from continued
breast-feeding
could result in a total effective dose equivalent exceeding 5
millisieverts (0.5 rem).
[62 FR 4133, Jan. 29, 1997]
REFERENCES:
(1) J Nucl Med 2001; Coover LR, et al. Therapeutic 131-I in outpatients: A simplified method conforming to the code of federal regulations, Tital 10, Part 35.75. 41: 1868-1875
(2) J Nucl Med 2001; de Klerk JMH. 131-I therapy: Inpatient or outpatient? 41: 1876-1878
(3) J Nucl Med 2012; Siberstein EB, et al. The SNNMI practice
guideline for therapy of thyroid disease with 131I
3.0*.
53: 1633-1651
(4) Radiology 2014; Liu B, et al. Thyroid cancer: radiation
safety precautions in 131I therapy based on actual
biokinetic measures. 273: 211-219