Normal Thyroid:
On ultrasound, the normal thyroid appears homogeneous echogenic
[43]. The thyroid lobes are normall 4-6 cm in craiocaudal length
and 1.3-1.8 cm in AP and transverse dimensions [43]. The isthmus
normall measures up to 3 mm [43].
Thyroid Nodules
Thyroid nodules occur in 4-15% of the adult population by palpation and in 10-41% by ultrasound [11,20]. The prevalence of thyroid nodules increases with age and can be found in up to 90% of women over the age of 60 years [11,20]. The vast majority of these nodules are benign- only 2-7% of all thyroid nodules, 6-9% of non-palpable nodules, and 9-13% of nodules selected for fine-needle aspiration are malignant [24,43]. The incidence of thyroid malignancy is only 50 per million population [10] and the lifetime risk for thyroid cancer is less than 1% for the U.S population [43].. The major challenge that faces a clinician is to determine whether a thyroid nodule is benign or malignant. Malignancy is more common in nodules found in patients who are younger than 20 years or older than 60 years [20]. A history of neck irradiation or a family history of thyroid cancer are also associated with asn increased risk for malignancy [20]. Although certain aspects of the history and physical examination may suggest an increased risk for malignancy, in most cases these are nonspecific and are of no predictive value. Additionally, the presence of multiple nodules (MNG) does not decrease the likelihood that one of them is a carcinoma, as was once thought [19,20,21]. Additional tests that may be helpful in establishing a diagnosis include thyroid function tests, thyroid scan, thyroid ultrasound and fine needle aspiration biopsy (FNAB).
A serum TSH level and thyroid function tests should be obtained for any thyroid nodule larger than 1-1.5 cm [21]. The thyroid function tests will reveal whether a patient with a thyroid nodule is euthyroid, hypothyroid, or hyperthyroid. If the TSH is suppressed, nuclear medicine imaging can be performed to document an autonomously functioning nodule [21]. Most patients with thyroid cancers are euthyroid, therefore hypothyroid (i.e. Hasimoto's disease) or hyperthyroid patients (autonomously hyperfunctioning nodules) with thyroid nodules are more likely to have a benign process. However, there are exceptions to this as patients with Graves' disease and a dominant cold nodule may have underlying thyroid cancer that behaves in a more aggressive manner. [1]. Hypothyroid patients with Hashimoto's disease and a dominant nodule or a rapidly enlarging goiter may also have lymphoma of the thyroid and require FNAB. Thyroid ultrasound can document the size of a thyroid nodule and provide an objective parameter for assessing response to hormone suppression.
Based upon their scintigraphic appearance, nodules can be classified as cold, hot or indeterminate (function, non-delineated, warm). The differential diagnosis for these nodules are described below.
Cold Nodule
A cold nodule demonstrates decreased tracer uptake compared to the surrounding normal thyroid tissue [7]. A cold nodule reflects lack of organification (or trapping if Tc-pertechnetate is the imaging agent) and subsequent thyroxine synthesis. The great majority of solitary thyroid nodules are cold (hypofunctioning), but only 10 to 25% of these are malignant [8]. Thyroid cancers appear as cold nodules due to altered iodine metabolism characterized by decreased iodine uptake and markedly reduced iodine organification [18].
Although some authors have reported a lower incidence of cancer in cold nodules in patients with multinodular goiter (1 to 6% risk of malignancy if the patient has a MNG), this is not confirmed in other reports. In a large review of patients presenting for the evaluation of a cold thyroid nodule, the frequency of thyroid cancer was about 5% (in an iodine sufficient area) and there was no change in the frequency of malignancy (4.9%) in patients with a multinodular goiter and a dominant nodule. [3,4]. In another article [11], the rate of malignancy in cold nodules in MNG was 9.8% in cold nodules and 8% in the single nodule group. Other authors have also concluded that there is not a statistically significant difference in the incidence of thyroid cancer in patients with solitary or multiple thyroid nodules [12,20]. The Society of Radiologists in ultrasound consensus statement is that the incidence of cancer in patients with a thyroid nodules selected for FNA biopsy is 9.2%-13%- no matter how many nodules are present [20]. The American Thyroid Association and the American Association of Clinical Endocrinologists recommend FNAB on dominant nodules in patients with MNG?s [11]. However, approximately one-third of thyroid cancers occur in non-dominant nodules [20]. The bottomline is that multinodularity of a goiter should no longer be considered an indicator of probable benign disease [11].
The likelihood of a cold nodule being malignant was lower in iodine deficient patients (roughly 2.5-3%) [3].
Differential considerations for a cold nodule
include:
Benign:
(Roughly 80% of cold nodules are secondary to benign leions [7])
1- Simple Cyst
True epithelial lined thyroid cysts are RARE. More often, cystic lesions detected in the thyroid represent degenerating adenomas or colloid nodules. Predominantly cystic lesions with a solid component (such as a wall nodule) are usually benign (85%).
2- Adenomatous hyperplasia:
(Colloid cyst/Non-functioning Follicular Adenoma)
A colloid cyst is a localized colloid filled follicle. It is the most common cause of a hypofunctioning thyroid nodule (60%). It may be solid, but areas of hemorrhage or cystic degeneration are commonly seen. Patients usually present with an enlarging thyroid nodule. Rapid enlargement and pain is associated with intralesional hemorrhage. On aspiration, the cyst fluid will have high T3 and T4 levels.
On ultrasound these lesions are typically a solid hypoechoic (70%) or complex lesion with a well defined hypoechoic rim or halo (such a halo is seen in only 10-15% of thyroid carcinomas.)
3- Focal
Hemorrhage
4- Inflammatory:
- Focal thyroiditis
- Abscess
5- Parathyroid
Adenoma
Malignant
(20%)
1- Thyroid
carcinoma
2- Parathyroid
adenoma/carcinoma
3- Thyroid Lymphoma
4- Metastatic
Disease
Riskfactors
for malignancy
Factors which increase the risk of malignancy in a cold nodule include:
1- History of XRT to head and neck as an adolescent or child: The likelihood of malignancy in a solitary nodule is about 30% if there is a history of XRT and 35% if multiple nodules are detected. It is important to note that about 5% of patients who received XRT in childhood and have a normal thyroid scan are found to have a malignancy.
2- Adenopathy (Regional)
3- Age: Less than 20
years (about 2 fold increased risk [3]) or over 60 years (about
6 fold increased risk [3]). Other authors suggest that the risk
for thyroid cancer increases when the patient is over the age of
45 years [43].
4- Male sex: The chance that a cold nodule is malignant is about 2 times greater in a male patient [3]. Generally, carcinoma is found in about 20-25% of cold nodules in men.
5- Evidence of local invasion: Recurrent laryngeal nerve involvement - hoarseness
6- Size of nodule greater than 4 cm
7- Nodule enlarges, especially while on thyroxine suppression: Mostbenign nodules will shrink or remain unchanged.
8- Family history of thyroid cancer
9- MEN 2 syndrome
Hot Nodule
A hot nodule has greater more activity than the normal surrounding thyroid tissue [7]. It can be autonomous (non-responsive to TSH manipulation), semiautonomous (partially responsive), or non-autonomous (responsive). An autonomous nodule will continue to function and show uptake of iodine even when TSH has been suppressed by administering exogenous thyroid hormone (refer to TSH suppression test). A toxic nodule is an autonomous nodule that produces enough thyroid hormone to cause thyrotoxicosis.
Differential considerations for a hot nodule
include:
Benign Thyroid
Lesions as Hot Nodules:
1- Benign hyperfunctioning follicular adenomas
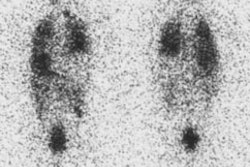
Accounts for almost all hot nodules, 50% are autonomous- i.e.: TSH independent. Patients can be euthyroid or hyperthyroid (Plummer's disease) as a result of the hyperfunctioning nodule. The remainder of the thyroid gland is suppressed with a toxic nodule, but can be imaged if TSH is given to stimulate this tissue. As these nodules enlarge, they frequently undergo central necrosis and may be centrally photopenic.
|
Autonomous Nodule: Pinhole images from an I-123 scan demonstrate an autonomously functioning nodule within the lower pole of the right lobe of the thyroid gland. The remainder of the thyroid is suppressed by this hyperfunctioning nodule. The patients radioactive iodine uptake was 27%. |
|
|
2- Adenomatous Hyperplasia
3- Compensatory Hypertrophy [7]
TSH dependent nodular hyperplasia with intervening fibrosis. Compensatory hypertrophy can cause a palpable nodule which concentrates pertechnetate better than the surrounding tissue [7]. Such hypertrophy is seen when there is widespread damage to the gland (Hashimoto's).
4- Physiologic Thyroid Hyperplasia
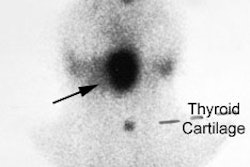
Patients who have congenital lobar agenesis (more commonly the left lobe [80%]), or are post surgical lobectomy, may appear to have a hot nodule which is suppressing the remainder of the gland.
Malignant
Thyroid Lesions as Hot Nodules:
1- Thyroid Carcinoma
EXTREMELY RARE. The probability of cancer in a hot nodule scanned with radioiodine in less than 4% [5].
Indeterminate
Nodule
An interminant , 'warm' or 'non-delineated' nodule has activity equal to the adjacent thyroid gland. One reason the lesion may not be identified is due to shine through of normal thyroid tissue activity. A thyroid suppression test may be performed to determine if the nodule is autonomous or cold. Cold nodules require further evaluation to exclude malignancy.
Discordant
Nodule
A discordant nodule is hot on Tc-99m images, but cold on the I-123 exam. Discordant nodules can be explained by either the preservation of Tc-pertechnetate trapping, but failure of organification or the rapid release of organified iodine from the nodule (iodine has washed out of gland by time of scanning at 24 hours) [6]. Solitary discordant thyroid nodules are generally considered to be rare (2 to 8%) and cases of discrepancy between the Tc-99m and I-123 studies appear most often in multinodular goiters [6,17]. Discrepancies are also far more likely to be caused by benign thyroid disorders rather than malignancy [2,6].
Although some authors feel that as many as 30% of discordant nodules may be malignant, earlier concern for discordant nodules has lessened. A conservative approach to this problem would be to re-scan any patient with a hot nodule on the Tc-99m pertechnetate exam with I-123. However, the risk of cancer in a nodule appearing hot with Tc-99m and cold with radioiodine is probably so low that routine reimaging is not necessary [4,6].
Thyroid nodule
ultrasound and fine needle aspiration biopsy:
Sonography has demonstrated that non-palpable thyroid nodules are 4 times more common than those which are detected clinically. The prevalence of nodules also increases with age [20]. Thyroid nodules are found by US in 10-50% of patients [12,15,20], but less than 7% of thyroid nodules are malignant [25]. However, the true malignancy rate in incidentally detected nodules may be lower, as this represents a subset of nodules that demonstrated suspicious features that warranted a biopsy (i.e.: the great majority of nodules are not biopsied) [42]. At post mortem evaluation, about 50% of patients are found to have thyroid nodules [20] and incidental small foci of thyroid cancer can be found in 36% of patients who died of other causes [42].
A contentious question for future research is whether treatment
of small papillary cancers improves survival [42]. The 10-year
survival rate for a 14-mm localized tumor is 99.6% following
treatment [42]. In a study of patients with papillary
microcarcinomas who did not receive treatment, after 10 years
new nodal metastases were found in 3% of cases and there had
been no cancer deaths [42].
The risk of malignancy in a thyroid with multiple nodules
is comparable to that with a single nodule [25,29].
A major dilemma in the evaluation of small
thyroid nodules detected at US is the determination of which
nodules should undergo fine needle biopsy. Some nodules will
not be evident on scintigraphy
due to their small size or superimposition of normal thyroid
tracer activity and therefore they cannot be accurately
classified as "hot" or "cold". The society of Radiologists in
Ultrasound suggests that FNA should be considered for a nodule
1 cm or more at the largest diameter if microcalcifications
are present and for a nodule 1.5 cm or larger if the nodule is
solid or if there are coarse calcifications within the nodule,
and for mixed solid-cystic nodules 20 mm or larger [29,42].
Using SRU criteria, up to 18% of incidentally cancers may not
meet criteria for biopsy [45]. However, the American
Association of Clinical Endocrinologists recommends FNA even
for nodules smaller than 10 mm whenever clinical
information or US features arouse suspicion for the
presence of malignancy (such as irregular margins, intranodular vascular spots,
taller/longer than wide shape, or microcalcifications)
[29,32]. Most nodules referred for fine needle biopsy are 1 cm
in size or greater and the American Thyroid Association also
recommends that generally, only nodules larger than 1 cm
should be evaluated as they have the potential to be
clinically significant cancer [21]. However, using size
criteria is not an adequate criteria
as cancer can occur in a significant number of nodules less
than 1 cm in size [12]. Inclusion of smaller nodules,
however, would lead to an excessive number of biopsies and there
is uncertainty that biopsy of nodules smaller than 1 cm improves
life expectancy [20] (a long term study has suggested no
difference in outcomes between patients with biopsy-proven
carcinomas < 1 cm undergoing thyroidectomy and those followed
with no surgical intervention [48]). None-the-less, FNA of
nodules less than 1 in size should be performed if the nodule
demonstrates features which increase the risk of malignancy or
if the patient has clinical risk factors for thyroid cancer
(prior head and neck irradiation or family history of thyroid
cancer) [20,21,22]. Another set of criteria is the three-tiered
system which calls for the workup of nodules with aggressive
imaging features (suspicious lymph nodes, local invasion, or
focal metabolic activity at PET); presence in a patient younger
than 35 years of age, and solid nodules 15 mm or larger [42].
The sonographic characteristics of
a nodule can be used to aid in suggesting whether a nodule is
benign or malignant and are superior to using size criteria
alone [20,21]. Hyperechoic
solid nodules are usually benign (96%) [15] (however,
note that sclerosing papillary neoplasms can also have this
appearance). A giraffe pattern has been described as a nodule/nodules with globular areas
of hyperechogenicity separated by
thin band-like areas of hypoechogenicity
producing a pattern similar to the two-tone block-like coloring
of a giraffe [30]. This pattern is characteristic of Hashimoto's
thyroiditis [30].
Mixed lesions represent solid lesions which have undergone
variable degrees of cystic degeneration. A predominantly cystic
lesion (greater than 75% with no calcification) has only a 1%
likelihood of being a cancer [20]. Completely
cystic lesions that are completely smooth walled and anechoic
are almost always benign. A honeycomb or spongiform
consistency (aggregation of multiple microcystic components more
than 50% of the volume of the nodule) is also suggestive of a
benign nodule [25- Invited commentary,27,30,35].
For partially cystic nodules, it is important to evaluate the
solid component [48]. If the solid component is eccentrically
(peripherally) located within a partially cystic nodule and the
margin of the solid component is has an acute angle with the
wall of the nodule, the risk for malignancy is increased [48].
Also- if the solid component is hypoechoic, lobulated, has an
irregular border, contains punctate echogenic foci, or has
vascular flow, the risk for malignancy is increased [48]. If the
solid component is isoechoic, centrally located within the
nodule, or peripheral, but smooth without acute angles with the
nodule wall, or a spongiform appearance, the nodule is likely
benign [48]. Punctate echos within a nodule with comet tail
artifacts (a reverberation artifact) are associated with bening
colloid nodules (although small, as opposed to large, comet tail
artifacts may be found in up to 15% of malignant nodules) [48].
Some authors suggest that a uniform hypoechoic halo is also
suggestive of a benign nodule [43].
Iso- or hypoechoic nodules may be benign or malignant.
US findings that suggest an increased risk for malignancy and aid in the identification of nodules that should undergo biopsy (even if less than 1 cm) include: [12,20,22,25,27]
1- Microcalcifications
(or coarse internal calcifications). Microcalcifications
appear as very small less than 1mm hyperechoic
foci that do not necessarily shadow [22,43]. They correspond
to psammomatous calcifications
[27]. Microcalcifications are
found in 29-59% of all primary thyroid caricnomas-
most commonly papillary cancer [25,43]. Microcalcifications
indicate malignancy in a thyroid nodule most successfully of
any single US feature [50]. The presence of microcalcifications in a predominatly solid nodule are associated
with a 3-4.5 fold increase cancer risk and coarse calcification
with a 2-2.5 fold increase [20,27]. The sensitivity of microcalcifications
for cancer is 29-59%, but the specificity is 86-98% (microcalcifications are one of the
most specific US findings of a thyroid malignancy [25]) [22,27,32,41]. The positive predictive
value for malignancy is 42-94% [25]. However, other authors
suggest microcalcifications have a sensitivity of 89%,
specificity of 95%, and accuracy of 94% [51]. The presence of
localized microcalcifications
without an associated discrete nodule should also prompt
biopsy as the finding has been described in association with
papillary carcinoma [26]. Large irregularly shaped
calcifications may occur secondary
to tissue necrosis [25] and are also associated with an
increased risk for malignancy (slightly mor than double the
baseline risk [48]) [27]. They are commonly present in multinodular goiters, but when seen in
a solitary thyroid nodule the malignancy rate can be as high
as 75% [25]. Coarse calcifications are also the most common
type seen in medullary carcinomas
[25].
Some authors have suggested that rim calcification of a nodule increases the risk for cancer by twofold [36] and this type of calcification can be seen with both papillary and medullary cancers [43]. In one study, 27% of nodules with peripheral rim calcification were found to be malignant [54]. Some authors have suggested that nodules which demonstrate discontinuous/interrupted peripheral calcification with nodule protrusions have a higher risk of malignancy, but this have not been shown in all studies [54]. A nodule that demonstrates posterior acoustic shadowing may also be associated with an increased risk for malignancy [38]. Inspissated colloid calcifications can be identified by their associated ring down or reverberation artifact (comet tail) and should not be confused with malignant calcifications [25].
2- Irregular, spiculated,
micolobulated, or blurred margins
[22]. Ill-defined and irregular margins are suggestive of
malignant infiltration of adjacent thyroid tissue [25]. A
thyroid nodule is considered ill-defined when more than 50% of
its border is not clearly demarcated [25]. The sensitivity of
this finding for cancer is 55-77.5%, and the specificity
83-85% [22,41]. However, other sensitivities have been
reported for ill-defined (53-89%), irregular (7-97%) [25,32], and spiculated
margins (48%) [27]. Note that between 33-93% of malignant
nodules will still have smooth borders [48].
3- Intranodular vascularity with a chaotic arrangement
of blood vessels related to arteriovenous
shunts and tortuous vessel course
greater than the adjacent thyroid tissue [12,20,22,25]. The sensitivity of this finding is
about 74%, and the specificity about 81% [22]. In a meta-analysis, intranodular vascularization had
a sensitivity of 40% and a specificity of 61% for malignancy
[41]. However, other authors report that vascularity
is frequently seen in benign nodules (more
than 50% of hypervascular nodules
can be benign [25]) and that the finding
is not useful for predicting thyroid malignancy [34]. Hypervascularity within the central aspect of the
nodule has also been indicated as suggestive of malignancy
[43]. A completely avascular
nodule is very unlikely to be malignant [25,43].
4- A solid very hypoechoic nodule: A hypoechoic nodule is less echogenic than the thyroid parenchyma, while a markedly hypoechoic nodule has less echogenicity than the adjacent strap muscle [43]. The combination of these features has a sensitivity of 78-87% for the detection of thyroid malignancy, but has low specificity (16-55%) and can be seen in 55% of benign nodules [25,41]. Using these criteria, hypoechoic nodules with at least one risk factor can identify 87% of cancers [12]. Marked hypoechogenicity may be a less sensitive finding as in two other studies, marked hypoechogenicity had a sensitivity 37-41% and a specificity of of 92-97% [27,32].
5- No well defined hypoechoic halo: A halo or hypoechoic rim around a thyroid nodule is produced by a pseudocapsule of fibrous connective tissue, compressed thyroid parenchyma, and chronic inflammatory infiltrate [25]. A completely uniform, thin halo is highly suggestive of a benign nodule (specificity of 95%) [25]. However, up to 10-24% of papillary thyroid cancers can have a complete or incomplete halo [25] and other authors recommend biopsy of these nodules [30].
6- Taller than wide nodule (greater in
the anteroposterior dimension than
its transverse dimension [28]) - indicates growth across normal
tissue planes [25,27]. This finding
is seen in about 12% of thyroid nodules [48]. Sensitivity
24-76%, specificity 60-91% (the sign is very specific, but not
sensitive for malignancy), positive predictive value 58-73%, and
negative predictive value between 77-88% [27,32,39,41,48,51].
7- A solid mass with refractive shadowing from the edges that is believed to occur as a result of fibrosis [30].
8- Interval growth: Interval growth is a poor indicator of
malignancy. Approximately 90% of nodules undergo a 15% or
greater increase in volume over 5 years [25].
9- Compressibility: An elasticity score is a visual scale
assigned to an ultrasound elastography color image according to
the degree and distribution of strain induced by light
compression [41]. A strain ratio is calculated from a ROI (mean
color pixel density) adjusted to lesion contours and a
comparable ROI placed in the adjacent tissue (either normal
thyroid, surrounding strap muscle, or tissue near the caroitd
arotid artery) [21]. Benign nodules are more compressible than
malignant nodules, however, it is difficult to apply a standard
amount of pressure and the technique is subject to interobserver
variability [39]. In one study evaluating nodule
compressibility, there was a sensitivity of 51% and a
specificity of 87% for the diagnosis of malignancy [39]. A
meta-analysis suggested sensitivities and specificities of 82%
and 82%, and 89% and 82% for elasticity score and strain ratio,
respectively [21]. However, other authors have found that
thyroid nodule elastography is less reliable than gray scale
features for differentiaiton of thyroid nodules [40].
The presence of abnormal lymph nodes (replaced fatty hilum, rounded bulging shape,
heterogeneous echotexture,
echogenicity greater than that of adjacent musculature,
microcalcifications, cystic areas (up to 70% of papillary cancer
node metastases can have a cystic component [25]), and vascularity peripherally or throughout
the node (instead of the normal central hilar
vessels) should also rise the level
of concern for malignancy within a thyroid nodule [20,51].
Microcalcifications and cystic degeneration have been reported
to have the highest specificity (up to 100%), whereas increased
peripheral vascularity has the highest combined sensitivity and
specificity (86% and 82%, respectively) [51]. The
presence of abnormal cervical lymph nodes should prompt FNA of
the nodes and ipsilateral thyroid
nodule of any size [20]. The presence of a fatty hilum
virtually excludes malignancy (sensitivity 100%, specificity
29%) [51].
Society of Radiologists in US Recommendations for Thyroid
Nodules 1 cm of Greater [20]:
|
US Feature |
Recommendation |
|
Microcalcifications |
Strongly consider
biopsy if greater than or equal to 1 cm |
|
Solid or almost
entirely solid or coarse calcifications |
Strongly consider
biopsy if greater than or equal to 1.5 cm |
|
Mixed solid and cystic
or almost entirely cystic with solid mural component |
Consider biopsy if
greater than or equal to 2 cm |
|
None of the above, but
substantial growth since prior US exam |
Consider biopsy |
|
Almost entirely cystic
and none of the above and no substantial growth |
Biopsy is probably
unnecessary |
|
Multiple nodules |
Consider US biopsy of
one or more nodules with selection based upon specific
criteria |
|
Multiple nodules |
Biopsy is likely
unnecessary in a diffusely enlarged gland with
multiple nodules of similar US appearance without
intervening parenchyma |
The ACR TIRADS
scoring system can be seen here which assigns nodules to
one of five ascending risk levels (TR1 to TR5) based on nodule
characteristics [56]. Studies have shown that a higher
percentage of malignancies (17-32%) do not receive a
recommendation for FNA when ACR TI-RADS is used compared to
guidelines from the American Thyroid Association (5-25%) [55].
Applying TI-RADS criteria to a large registry of thyroid nodules
resulted in a biopsy recommendation for 26% of nodules, while
applying ATA criteria resulted in a biopsy recommendation in 51%
of nodules [56]. This results in a lower sensitivity and higher
specificity for TI-RADS (53-69%) compared to the ATA guideines
(specificity 22-45%). None-the-less, some authors have suggested
decreasing the threshold for a TI-RADS TR5 nodule FNA from 1 cm
to 5 mm [55].
If appropriate expertise is available, fine needle aspiration
of a thyroid nodule is the most cost effective management [2]. For fine needle aspirates considered sufficient for
diagnosis, the sensitivity and specificity are 93% (76-98%
[29]) and 96% (71-100% [29]), respectively [15]. The
overall accuracy is between 69-97% [29]. FNAB has decreased the
need for thyroid surgery by about 50% and increased the yield of
cancer in excised thyroid nodules by about 40%. About 95% of
nodules that are biopsied are benign [19] and the risk of
malignancy in a nodule reported as benign at FNA is between 0-3%
[43]. A problem with FNA of thyroid nodules is
that up to 10-25% of thyroid nodule biopsies can result
in inadequate specimens/unsatisfactory cytologic
analysis [9,12,19,29].
Cystic nodules are more likely to yield unsatisfactory
results [9]. Benign nodules also have a higher
chance of being inadequate for cytologic
diagnosis than malignant nodules [29]. Between 3.1% to
10% of nodules with non-diagnostic aspirates may be malignant
(other authors indicate the risk as being between 2-51% [37])
and repeat biopsy should be considered in those cases [9,29,49]. For nodules with non-diagnostic
biopsy results, the risk for malignancy is related to the
presence or absence of suspicious features [46]. The ATA
guidelines suggest that non-diagnsotic thyroid nodules with
very-low or low suspicion US features can be monitored with
followup US [53]. However, nodules with intermediate or high
suspicion features, should undergo re-biopsy [53]. The risk for
malignancy can be as high as 31-47% if there are multiple
suspicious features present [46,53]. A repeat biopsy is
diagnostic in 50% of cases [29], although, other authors
indicate that inadequate cytology occurs in 25-67% of repeat
biopsies [37]. Several authors recommned that a repeat biopsy
not be performed until 3 months after the initial biopsy to
prevent false positive findings related to transient reparative
cellular atypia [43,46].
False negative biopsy results can occur in 1 to 6% of cases and
a patient with a benign FNA has a 4-6% chance of ultimately
proving to have a cancer [14,31,44].
The appearance of the nodule affects the likelihood of a
false-negative diagnosis [29,31,46].
Among nodules initially diagnosed as benign, the rate of
malignancy has been reported to be 0.6-2.1% for nodules with
benign US features and as high as 13.6-20% if the nodule has
suspicious US features [29,31]. Thus,
it is reasonable to repeat the FNA if the biopsy
result in negative, but the nodule has one or more
suspicious US features [31,47]. For nodules with benign features
and a initial negative FNA, an
increase in size over time is associated with a 1.4% risk of
malignancy [31]. Therefore, it has been recommended to observe
all thyroid nodule patients even with a benign FNA result to
ensure stability over time [14,21].
For nodules with two negative FNA results, there is a 98-100% likelihood for a benign nodule
[31]. Follicular variant of papillary carcinoma can have
relatively benign US features such as well-defined margins and
no microcalcificaitons, and it is also less likely to have a
taller-than-wide shape compared to papillary carcinomas [44].
Circumstances that necessitate repeat FNA include nodule
enlargement, cyst recurrence, or clinical/imaging
findings that arouse a suspicion for the presence of malignancy
[29]. The American Thyroid Association recommends repeat US
guided FNA following a benign result for nodules with highly
suspicious features (for nodules with a low to intermediate
suspicion, a repeat US in 12-24 months is recommended) [52].
Growth of the nodule should then prompt re-biopsy (growth= a 15%
increase in nodule volume or a 20% increase in nodule diameter
with a minimum increase in 2 or more dimensions of at least 2
mm) [21,31]. The American Thyroid
Association guidelines suggest that a 50% increase in nodule
volume on follow-up performed 6 to 18 months after intial FNA
should prompt rebiopsy [44] or if there is the interval
development of new suspicious sonographic features [52].
However, even for nodules demonstrating a 50% increased in
volume, repeat FNA is unlikely to reveal malignancy unless the
nodules displays suspicious features [44]. In one followup study
of patients with an initial benign biopsy result, ultimately
only 2.4% of nodules eventually proved to be cancer [52]. At
least 3 months should be allowed to elapse after the initial FNA
prior to re-biopsy- this time will avoid problems in cytologic interpretation that may be
posed by reparative cellular atypia
that could be mistaken for malignancy [29]. Note that lack of
change in size over time does not exclude malignancy, especially
if the nodule has suspicious features [44].
False positive biopsy results can occur in 0 to 5.7% of biopsies [29,43] (although higher rates of 7.4% to 25% have been suggested). This is mostly a problem for lesions described as ?suggestive of follicular neoplasm? [11] because follicular adenomas and carcinomas cannot be distinguished cytologically [19]. Among solitary thyroid nodules with an indeterminate biopsy result, the risk of malignancy is about 10-20% (i.e.- 80-90% of the lesions will prove to be adenomas) [21,33].
In patients found to have thyroid malignancy following fine needle biopsy, no needle tract implants occurred in an early series of 1,400 needle biopsies and it is a rarity in other series [14].
Sonographically guided percutaneous ethanol injection is an alternative to surgery for patients with symptomatic non-functioning benign thyroid cystic or solid nodules [16].
CT and thyroid nodules:
Incidental thyroid nodules are commonly seen on CT imaging, however, CT commonly underestimates the number of nodules compared to US [23]. In a sub-selected patient population, almost 4% of nodules found at CT are malignant [23]. Patients under the age of 35 with incidental thyroid nodules and nodules larger than 2.5 cm have a greater risk for malignancy [23].
REFERENCES:
(1) J Clin Endoc Metab 1990; Belfiore A et al. Increased aggressiveness of thyroid cancer in patients with Graves' disease. 70:830-835
(2) Semin Nucl Med 1994; Freitas JE, Freitas AE. Thyroid and parathyroid imaging. 24: 234-245
(3) American Journal of Medicine 1992; Belfiore A, et al. Cancer risk in patients with cold thyroid nodules: Relevance of iodine intake, sex, age, and multinodularity. 93: 363-69
(4) Semin Nucl Med 1995; Dworkin HJ, et al. Advances in the management of patients with thyroid disease. 25: 205-220
(5) Radiol Clin North Am 1993; Price DC. Radioisotopic evaluation of the thyroid and the parathyroids. 31: 991-1015
(6) J Nucl Med 1990; Kusic Z, et al. Comparison of technetium-99m and iodine-123 imaging of thyroid nodules: Correlation with pathologic findings. 31: 393-99
(7) Endocrinology and Metabolism Clinics of North America 1990; Shulkin BL, Shapiro B. The role of imaging tests in the diagnosis of thyroid carcinoma. 19: 523-541
(8) Thyroid and whole-body imaging. Charkes ND. In The Thyroid, 5th ed. Ed Ingbar and Braverman. Lippincott, Philadelphia, 1986. 458-478
(9) Radiology 2002; O'Malley ME, et al. US-guided fine-needle aspiration biopsy of thyroid nodules: adequacy of cytologic material and procedure time with and without immediate cytologic analysis. 222: 383-387
(10) Radiology 2003; Screaton NJ, et al. US-guided core-needle biopsy of the thyroid gland. 226: 827-832
(11) Endocr Pract 2000; Sachmechi I, et al. Thyroid carcinoma in single cold nodules and in cold nodules of multinodular goiters. 6: 5-7
(12) J Clin Endocrinol Metab 2002; Papini E, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-doppler features. 87: 1941-1946
(13) Arch Surg 2002; Blansfield JA, et al. Recent experience with pre-operative fine-needle aspiration biopsy of thyroid nodules in a community hospital.
(14) J Surg Oncol 2002; Lawrence W, Kaplan BJ. Diagnosis and management of patients with thyroid nodules. 80: 157-170
(15) Southern Journal of Medicine 2002; Supit E, Peiris AN. Cost-effective management of thyroid nodules and nodular goiters. 95: 514-519
(16) AJR 2003; Kim JH, et al. efficacy of sonographically guided percutaneous ethanol injection for
treatment of thyroid cysts versus solid thyroid nodules. 180:
1723-1726
(17) Radiographics 2003; Intenzo CM, et al. Scintigraphic manifestations of thyrotoxicosis. 23: 857-869
(18) J Nucl Med 2005; Robbins RJ, et al. The evolving role of 131I for the treatment of differentiated thyroid carcinoma. 46: 28S-37S
(19) N Engl J Med 2005; Utiger RD. The multiplicity of thyroid nodules and carcinomas. 352: 2376-2378
(20) Radiology 2005; Frates MC, et al. Management of thyroid nodules detected at US: society of radiologists in ultrasound consensus conference statement. 237: 794-800
(21) Thyroid 2006; Cooper DS, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. 16: 1-25
(22) Endocr Pract 2006; Gharib H, et al. American association of clinical endocrinologists and associazone medici endocinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. 12: 63-102
(23) AJR 2006; Shetty SK, et al. Significance of incidental thyroid lesions detected on CT: correlation among C, sonography, and pathology. 187: 1349-1356
(24) J Nucl Med 2007; Schoder H, Gonen M. Screening for cancer with PET and PET/CT: potential limitations. 48: 4S-18S
(25) Radiographics 2007; Hoang JK, et al. US features of thyroid malignancy: pearls and pitfalls. 27: 847-865
(26) AJR 2007; Kwak JY, et al. Papillary thyroid carcinoma manifested solely as microcalcifications on sonography. 189: 227-231
(27) Radiology 2008; Moon WJ, et al. Benign and malignant thyroid nodules: US differentiation- multicenter retrospective study. 247: 762-770
(28) AJR 2008; Kwak JY, et al. Thyroid incidentalomas identified by 18F-FDG PET: sonographic correlation. 191: 598-603
(29) Radiographics 2008; Kim MJ, et al. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. 28: 1869-1889
(30) AJR 2009; Bonavita JA, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? 193: 207-213
(31) Radiology 2010; Kwak JY, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. 254: 292-300
(32) AJR 2010; Ahn SS, et al. Biopsy of thyroid nodules: comparison of three sets of guidelines. 194: 31-37
(33) AJR 2010; Sillery JC, et al. Thyroid follicular carcinoma: sonographic features of 50 cases. 194: 44-54
(34) Radiology 2010; Moon HJ, et al. Can vascularity at power doppler US help predict thyroid malignancy. 255: 260-269
(35) AJR 2011; Virmani V, Hammond
I. Sonographic patterns of benign
thyroid nodules: verification at our institution. 196: 891-895
(36) Radiology 2011; Kwak JY, et al. Thyroid imaging reporting
and data system for US features of nodules: a step in
establishing better stratification of cancer risk. 260: 892-899
(37) AJR 2011; Kim DW, et al. Role of ultrasound diagnosis in
assessing and managing thyroid nodules with inadequate cytology.
197: 1213-1219
(38) AJR 2011; Sharma A, et al. Subcentimeter thryoid nodules:
utility of sonographic characterization and ultrasound-guided
needle biopsy. 197: 1442
(39) AJR 2012; Seo YL, et al. Compressibility of thyroid
masses: a sonographic sign differentiating benign from malignant
lesions? 198: 434-438
(40) Radiology 2012; Moon HJ, et al. Diagnostic performance of
gray-scale US and elastography in solid thyroid nodules. 262:
1002-1013
(41) AJR 2013; Razavi SA, et al. Comparative effectiveness of
elastographic and B-mode ultrasound criteria for diagnostic
discrimination of thyroid nodules. 200: 1317-1326
(42) AJR 2013; Hobbs HA, et al. Incidental thyroid nodules
detected at imaging: can diagnostic workup be reduced by use of
the Society of Radiologists in Ultrasound recommendations and a
three-tiered system? 202: 18-24
(43) Radiographics 2014; Nachiappan AC, et al. The thyroid:
review of imaging features and biopsy techniques with
radiologic-pathologic correlation. 34: 276-293
(44) Radiology 2014; Kim SY, et al. Thyroid nodules with benign
findings at cytologic examination: results of long-term
follow-up with US. 271: 272-281
(45) Radiology 2014; Bahl M, et al. Thyroid cancers
incidentally detected at imaging in a 10-year period: how many
cancers would be missed with use of the recommendations from the
society of radiologists in ultrasound? 271: 888-894
(46) Radiology 2015; Moon HJ, et al. Malignancy risk
stratification in thyroid nodules with nondiagnostic results at
cytologic examination: combination of thyroid imaging reporting
and data system and the Bethesda system. 274: 287-295
(47) Radiology 2015; Yoon JH, et al. Thyroid nodules:
nondiagnostic cytology results according to thyroid imaging
reporting and data system before and after application of the
Bethesda system. 276: 579-587
(48) J Am Coll Radiol 2015; Grant EG, et al. Thyroid ultrasound
reporting lexicon: white paper of the ACR thyroid imaging,
reporting, and data system (TIRADS) committe. 12: 1272-1279
(49) AJR 2016; Moon HJ, et al. Repeat ultrasound-guided
fine-needle aspiration for thyroid nodules 10 mm of larger can
be performed 10.7 months after initial nondiagnsotic results.
206: 823-828
(50) AJR 2016; Zayadeen AR, et al. Retrospective evaluation of
ultrasound features of thyroid nodules to assess malignancy
risk: a step toward TIRADS. 207: 460-469
(51) Radiographics 2016; Kumbhar SS, et al. Why thyroid
surgeons are frustrated with radiologists: lessons learned from
pre- and postoperative US. 36: 2141-2153
(52) AJR 2017; Becker-Weidman DJS, et al. Imaging surveillance
in patients after a benign fine-needle aspiration biopsy of the
thyroid: associated cost and incidence of cancer. 208: 358-361
(53) AJR 2018; Park CJ, et al. Thyroid nodules with
non-diagnostic cytologic results: follow-up management using
ultrasound patterns based on the 2015 American Thyroid
Association guidelines. 210: 412-417
(54) AJR 2019; Malhi HS, et al. Peripheral thyroid nodule
calcifications on sonography: evaluation of malignant potential.
213: 672-675
(55) AJR 2021; Middleton WD, et al. Analysis of malignant
thyroid nodules that do not meet ACR TI-RADS criteria for
fine-needle aspiration. 216: 471-478
(56) AJR 2021; Hoang JK, et al. Update on ACR TI-RADS
successes, challenges, and future directions, from the AJR
special series on radiology reporting and data systems. 216:
570-578