Tc-99m-Arcitumomab (CEA-Scan)
Arcitumomab (CEA-Scan) is a murine monoclonal Fab' fragment, generated from IMMU-4 directed against the CEA surface antigen found on colorectal carcinomas. The agent has a short biological half-life (13.4 hours) and rapid blood clearance, which results in improved target-to-background ratios. Pharmacokinetic studies revealed blood levels of 63%, 23%, and 7% of the injected dose at 1, 5 and 24 hours after infusion, respectively.
The small fragment size enhances renal clearance, and 28% of the agent is excreted in the urine over the first 24 hours after administration. Minimal liver metabolism permits improved hepatic imaging when compared to OncoScint. Optimal imaging can be accomplished within 5 hours of administration, providing same day information for clinical determinations.
Imaging is not affected by serum CEA levels [4], although the agent will form complexes with circulating CEA. Circulating human antimouse antibodies (HAMA) in patients that have previously received murine-based products may increase the risk of allergic reaction and potentially alter clearance and biodistribution.
CEA-Scan undergoes primary renal excretion, and intense renal activity is commonly encountered. There is less hepatic uptake of the agent compared to OncoScint; however, high renal activity may offset this advantage. If late imaging is performed (up to 24 hours post-injection), intestinal and gallbladder activity may interfere with imaging. Occasionally, bowel and gallbladder activity also may be visualized on the early images. Bowel preparation with an iso-osmotic solution and intravenous application of lasix are possible methods to overcome these disadvantages [3]. False positive examinations have been described in areas of inflammation, with colonic adenomas [6], and mis-interpretation of urinary activity [6]. A foley catheter in the urinary bladder can be used to clear urinary activity.
Hepatic metastatses are usually seen as "hot" or "hot-rimmed" lesions on images acquired later than 2-3 hours following injection. Liver metastases may appear as photopenic lesions on images acquired before this time, as the agent is still predominantly in the blood pool phase. Excretion of metabolites into the gastrointestinal tract can sometimes obscure recurrence at the anastomotic site. Cathartic agents are sometimes required to clear this activity.
1.25 mg of CEA-Scan is labelled with 25-30 mCi of Tc-99m, and infused with 30 ml of saline over a 5 to 20 minute period. The agent should be used within 4 hours of preparation. Prior to patient administration, radiochemical purity must be 90% by Instant Thin Layer Chromatography (ITLC).
Click here for the reommended imaging protocol
At the time of diagnosis, colorectal carcinoma is localized in only 36% of patients; regional lymph node metastases are present in 39% and distant mets are present in 19% [3]. Up to 40-50% of patients deemed surgical cures, will develop recurrence- most likely due to the presence of "micrometastases" that were not observed during their initial surgery [7]. Early detection of potentially resectable metastases or tumor recurrence leads to improved survival [2]. Serum levels of carcinoembryonic antigen (CEA) may be used to monitor the presence of recurrences with a sensitivity of 59% and a specificity of 84% [3]. The agent is not indicated as a screening tool for colorectal cancer.
Staging colorectal carcinoma:
For the staging of colorectal carcinoma the sensitivity of CEA-Scan has been shown to be superior to conventional imaging modalities in evaluation of the extra-hepatic abdomen (55% vs 32%) and pelvis (69% vs 48%). CEA-scan findings are not superior to conventional exams in evaluation of the liver (63% vs 64%); however, the findings are often complementary. Lesion detection is in part related to lesion size, with a sensitivity of 80% for lesions over 2 cm. Detection of lesions smaller than 1 cm is 60%, but this is still fairly good. CEA-Scan results can provide a potential clinical benefit in one-third of patients. CEA scan can also be injected 24-48 hours prior to surgery and by using hand-held intra-operative probes, surgeons can confirm tumor free margins and evaluate lymph nodes for the presence of metastases [7]. Any node with a count rate 1.5 times higher than healthy nodes (or a count rate greater than 25 counts per 10 seconds) should be considered suspicious and resected [7].
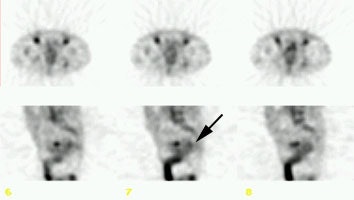
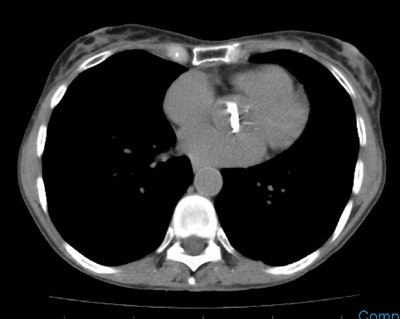
Staging colorectal carcinoma: The patient shown below had a large rectal cancer (white arrows on CT scan) and the CEA scan was done for staging purposes to assess for metastatic disease. Tracer uptake can be seen in the primary lesion (black arrow posterior to a small amount of urinary bladder activity), but no other sites of abnormal activity were identified. |
|
Recurrent colorectal carcinoma:
Isolated locoregional recurrence accounts for an estimated 25% of cases of initial spread of colorectal carcinoma [6]. Approximately 15-20% of these patients will be amenable to curative resection [6]. CEA-Scan can be used to detect recurrent or metastatic colorectal carcinoma in post-operative patients with a rising serum CEA, often in conjunction with standard imaging modalities. CEA scan has been shown to have excellent results for the detection of locally recurrent colorectal carcinoma (ie: distinguishing scar tissue from tumor recurrence) [3,5]. Sensitivity has been reported up to 89% for detection of local recurrence (compared to 33% for CT) [5]. Sensitivity for detection of recurrent cancer with lymph node metastases has been reported to be up to 71%, compared with 43% for CT [5]. Small lymph nodes (under 1.5 cm in size), may not be accurately identified [5]. CEA-scan alone or combined with CT has been found to be significantly more accurate for predicting non-resectability than CT alone [6]. The addition of CEA-scan to CT doubles the number of patients who should not undergo salvage surgery and increases the number of patients that are potentially resectable for cure by 40% [6]. When CT and CEA-scan findings are discordant, CEA-scan has been shown to be correct substantially more often than CT [6].
Surveillance CEA scanning following surgery for rectal cancer has also been shown to detect recurrence at an earlier stage than conventional imaging modalities [5]. This results in earlier, potentially curative re-operation with resultant improved long term patient survival [5].
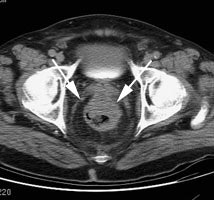
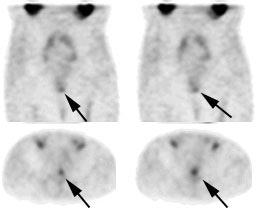
Recurrent colorectal carcinoma: The patient had prior resection for a rectal carcinoma and presented with a rising CEA. The CT scan demonstrated a soft tissue abnormality posterior to the urinary bladder that was interpreted as post operative scarring (white arrows). The CEA scan demonstrated a focal abnormality in the pelvis (black arrows) corresponding to the CT finding. At surgery this proved to be recurrent rectal carcinoma. |
|
Click here to view other CEA-scan cases
Minor adverse reactions such as nausea, urticaria, puritis, and fever have been uncommonly reported. More serious side effects are rare. Although anaphylactic reactions have not been observed to date, such reactions could occur. The agent should not be administered to patients with a history of hypersensitivy reactions to products of murine origin.
Unlike OncoScint, which is a complete IgG antibody, CEA-Scan is an antibody fragment, which produces less immunogenicity. As a result, formation of Human Anti-Mouse Antibody has been identified in fewer than 1% (0.3% [5]) of patients who have received the agent. Multiple injections do not appear to increase the risk for HAMA formation.
REFERENCES:
(1) J Clin Oncol 1996; Moffat FL, et al. Clinical utility of external immunoscintigraphy with IMMU-4-technetium-99m Fab' antibody fragment in patients undergoing surgery for carcinoma of the colon and rectum: MResults of a pivotal, phase III trial. The immunomedics study group. 14: 2295-2305
(2) Semin Nucl Med 1993; Podoloff DA, et al. Imaging of colorectal carcinoma with technetium-99m radiolabeled Fab' fragments. 23: 89-98
(3) J Nucl Med 2000; Willkomm P, et al. FDG PET and immunoscintigraphy with 99mTc-labeled antibody fragments for detection of recurrence of colorectal carcinoma. 41: 1657-1663
(4) Radiol Clin North Am 1997; Thoeni RF. Colorectal cancer. Radiologic staging. 35: 457-485
(5) J Am Coll Surg 2000; Lechner P, et al. Can postoperative surveillance with serial CEA immunoscintigraphy detect resectable rectal cancer recurrence and potentially improve tumor-free survival? 191: 511-518
(6) Annals of Surgery 1997; Hughes K, et al. Use of carcinoembryonic antigen radioimmunodetection and computed tomography for predicting resectability of recurrent colorectal carcinoma. 226: 621-631
(7) Cancer Research 2000; Lechner P, et al. Probe-guided surgery for colorectal cancer. 157: 273-280