J Endocrinol Invest 2002 Jan;25(1):44-52
Radioiodine ablation and therapy in differentiated thyroid cancer under
stimulation with recombinant human thyroid-stimulating hormone.
Berg G, Lindstedt G, Suurkula M, Jansson S.
We investigated whether recombinant human TSH (rhTSH) safely and effectively
induces uptake of high-dose 131-iodine (131I) to ablate thyroid remnant or treat
disease, in patients with well-differentiated thyroid carcinoma. Eleven
consecutive patients unable to tolerate thyroid hormone withdrawal received one
im injection of 0.9 mg rhTSH on 2 consecutive days before receiving 4000 MBq
(approximately 108 mCi) radioiodine orally. Eight patients received one, and 3
patients 2 courses. Our series comprised 7 women and 4 men (mean age, 78 yr,
range: 56-87 yr). Ten patients had undergone total or near-total thyroidectomy
up to 19 yr earlier. rhTSH-stimulated single course radioiodine with the
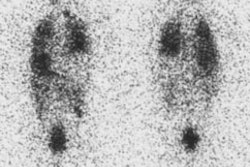
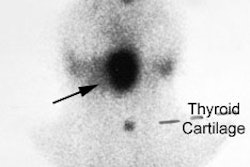
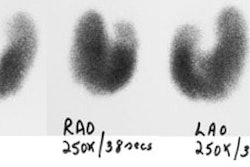
intention to ablate thyroid remnant was performed in 3 patients, with following
estimation of radioiodine uptake and TG measurements. Of another 8 patients
given this treatment palliatively, 5 had radiological, clinical and/or
laboratory response, including: 80% decreased pathological uptake between
treatment courses; pronounced decrease in bone pain; diminished symptoms;
improved physical condition and quality of life; lower serum TG concentration;
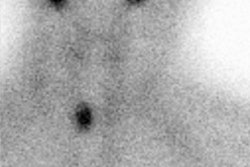
and/or normalization of TG recovery test. Two patients with small lung
metastases on computed tomography had no detectable radioiodine uptake or other
response; they also lacked uptake after withdrawal-stimulated radioiodine
treatment. Despite being elderly and frail, patients generally tolerated
treatment well; rhTSH caused nausea in one patient and transiently increased
pain in bone and soft tissue lesions in another. We conclude that rhTSH-stimulated
high-dose radioiodine for remnant ablation or tumor treatment is safe, feasible
and seemingly effective, enhancing quality of life and offering reasonable
palliation in patients with advanced disease.