Although he gained fame as a villain vanquished by Batman, Mr. Freeze was initially a women's health advocate -- of sorts. The icy bad guy started out as Dr. Victor Fries whose beloved wife Nora fell terminally ill. Fries then put her in cryostasis until a cure could be found to save her. During a laboratory scuffle, Fries wound up covered in cryogenic chemicals, resulting in his inability to survive above subzero temperatures, but endowing Mr. Freeze with the power to put people and things in an equally frigid state.
While Dr. Fries comic book-based life may have taken a turn for the worst from there, real-life breast health specialists are carrying on with his original intention by using cryoablation (or cryotherapy) to treat fibroadenomas, one of the most commonly diagnosed benign breast tumor, especially in women in their 20s and 30s.
This method of destroying diseased tissue by applying cycles of freezing and thawing temperatures has been most commonly used for the treatment of renal tumors, prostate cancer, and endometriosis. For breast fibroadenoma, ultrasound is used to guide the cryoprobe, through which argon gas flows, and an ice ball forms at the tip engorging and then freezing the tumor.
 |
 |
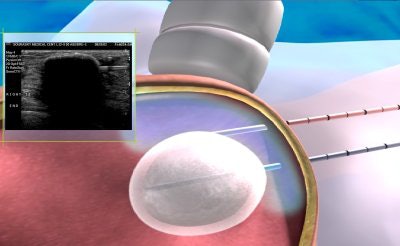
| Above, placement of cryoablation needle in the fibroadenoma. Middle, the ice ball forms at tip of cryoablation needle. Below, an illustration of ultrasound visualization of the ice ball. Images courtesy of Galil Medical. |
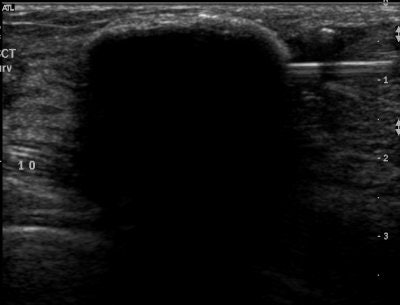 |
Proponents of cryotherapy for fibroadenomas point out that there is a growing demand and interest in minimally invasive therapies by patients as well as healthcare professionals. The Los Angeles-based University of Southern California (USC) Keck School of Medicine currently offers an 18-week, "live" breast clinic to teach such techniques as cryoablation (American Journal of Surgery, October 2006, Vol. 192:4, pp. 439-443).
Ultrasound-based breast procedures improve accuracy and efficiency, expand treatment options, and expand billable services "at a time of rising practice expenses and declining reimbursements," said Dr. Dennis Holms, director of new technology development and a breast surgeon at the USC center.
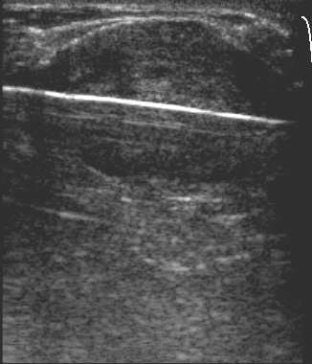 |
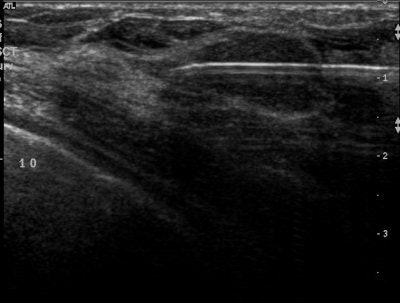
| Ultrasound images during cryoablation showing an ice-ball formation with a cryoprobe through the center of fibroadenoma. Above, longitudinal axis; below, transverse axis. Images courtesy of Dr. Dennis R. Holmes, breast surgeon and director of new technology development, University of Southern California/Norris Comprehensive Cancer Center and Hospital, and chief, breast service, LAC+USC Medical Center, Los Angeles. |
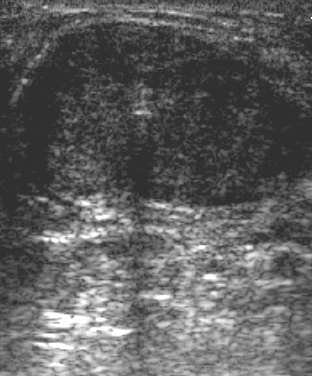 |
"When I first learned about the procedure, I thought it would be a great technique to treat women with fibroadenoma," said Dr. Jennifer Glass, chief of surgery at Women and Infants Hospital in Providence, RI. Glass estimated that she's treated about 10 to 20 cases to date. "It was as powerful a tool as I had hoped."
For the moment, breast surgeons are working with cryotherapy the most, but imaging specialists should take a closer look at this technique as an area where they broaden their practice by offering a noninvasive treatment alternative to patients.
Freeze, fibroadenoma!
In 1841, English physician Dr. James Arnott first used an iced salt solution to zap accessible cancers and found that the process reduced tumor size and relieved pain. Over time, better freezing materials became available -- solidified carbon dioxide, liquid nitrogen, nitrous oxide -- but cryotherapy was still rarely used. Automated cryosurgical units using liquid oxygen were introduced in 1961, but the inability to control the freezing process and bulky tools limited their usefulness. A renewed interest in cryoablation followed after the introduction of modern cryoprobes that applied the Joule-Thompson effect in which the temperature of a gas is either decreased or increased by letting the gas expand freely (Seminars in Surgical Oncology, December 7, 1998, Vol. 14:12, pp. 99-109).
The U.S. Food & Drug Administration (FDA) approved cryoablation as a treatment for biopsy-proven breast fibroadenomas about five years ago. Several new devices, from cryoprobe needles to sensor systems and handheld biopsy devices, have received FDA approval this year. Advocates of cryotherapy in this setting have enthusiastically pointed out its advantages:
- Minimal to no scarring
- Outpatient procedure that can be performed in less than an hour
- No general anesthesia
- Short recovery time
- Ability to treat multiple and recurring lesions
Dr. Peter Littrup and co-investigators at the Wayne State University School of Medicine in Detroit performed an overall assessment of percutaneous ultrasound-guided cryoablation in 29 patients with biopsy-confirmed fibroadenomas. According to their results, there was a 73% reduction in fibroadenoma volume size after treatment from a pretreatment average of 4.2 cm3 ± 4.7 to 0.7 cm3 ± 0.8.
 |
| US images. Above, transverse view of developing ice ball (< 1 cm in diameter; straight arrows) in 2.3-cm-diameter fibroadenoma (arrowheads) at initiation of freeze. Thermocouple tip (curved arrow) is 7 mm from the cryoprobe. Below, transverse view of growing ice ball (straight arrows) at two (left) and three (right) minutes, and thermocouple (curved arrow) is just becoming engulfed in ice at 6° C and -6° C, respectively. |
 |
"US produced excellent ice visualization beyond tumor margins, while thermocouples confirmed cytotoxic temperatures approximate 5 mm behind the visible leading edge," Littrup's group wrote. In addition, they noted that all but seven patients were able to tolerate the procedure under local anesthesia (Radiology, January 2005, Vol. 234:1, pp. 63-72).
In an e-mail to AuntMinnie.com, Littrup, who is also with the Detroit-based Karmanos Cancer Institute, updated his group's work in this area. He said that they are getting ready to publish their experience with cryotherapy in treating breast cancer and called the technique "ideal," especially in scattered recurrent metastases.
In the unpublished study, "we have limited-cancer patients to locally advanced cancer, chest wall recurrences, and isolated patients refusing lumpectomy," Littrup stated. "So far, all are pleased (with cryotherapeutic results) and (are) without recurrence, including the woman with (breast) implants."
 |
| Same patient as above. At three minutes, note the thickened skin distance (bracket) due to interval saline injection. Transverse view of a large unusually shaped (i.e., discoid, not cylindrical) fibroadenoma with cryoprobes (straight arrows) in place (top) and growing ice at one minute (bottom). The thermocouple (curved arrow) is about to be engulfed in ice. The ice ball subsequently fused to create a smooth discoid shape, which was only possible with the probes placed less than 1.5 cm apart and approximately 1 cm from the fibroadenoma margin. Figure 1a,c,d. Littrup PJ, Freeman-Gibb L, Andea A, et al. "Cryotherapy for breast fibroadenomas," Radiology 2005; 234(1):63-72. |
Littrup said his group has performed more than 400 cryoablation procedures in the breast, liver, lung, kidney, bone, and soft-tissue tumors. "It's therefore no longer a question of whether cryo kills any cancer, but how we best plan the cytotoxic isotherms and control with accurate image guidance."
A 37-patient study by Dr. Cary Kaufman and colleagues at the Bellingham Breast Center in Bellingham, WA, looked at long-term results after treatment and found that lesion volumes were reduced an average of 99% based on ultrasound exams up to three years postsurgery. The authors reported that 100% of the referring physicians and 97% of patients said that they were satisfied with the long-term results (Breast Journal, September-October 2005, Vol. 11:5, pp. 344-350).
At the same time, findings from the FibroAdenoma Cryoablation Treatment (FACT) registry were released. FACT included 55 U.S. practice sites with baseline and follow-up clinical data at six months and one year tabulated for all patients. According to the early results from 444 treated fibroadenomas, lesions continued to shrink over time, with tumor palpability reduced to 46% at six months and 35% at 12 months after the procedure. But results in women with larger lesions (3 cm or more) did not fare as well as those with smaller lesions (2 cm), they acknowledged. In addition, they said that it could take up to a year for a fibroadenoma to resolve completely (American Journal of Surgery, October 2005, Vol. 190:4, pp. 647-651).
How cryotherapy measures up
Besides the watchful waiting approach with ultrasound (fibroadenomas often resolve on their own), needle biopsy and surgical excision has been the traditional treatment for fibroadenomas. While these invasive procedures do offer the option of tumor removal, they come with a high incidence of scaring and ductal damage.
Another treatment avenue is thermal ablation, which requires sedation or general anesthesia. The outcomes of thermal ablation methods, including microwave, high-intensity ultrasound, radiofrequency, and laser ablation, are dependent on the ability to control blood flow.
In a meta-analysis, Dr. Pat Whitworth, director of the Nashville Breast Center in Tennessee, and John Rewcastle, Ph.D., at the University of Calgary in Alberta, Canada, reviewed cryoablation techniques along with other therapies. "Natural analgesic properties make cryoablation potentially more patient-friendly than other ablative technologies which raise tissue temperature. As a primary therapy for breast fibroadenoma, cryoablation is safe and effective with durable results that can be reproduced outside of the investigational setting," they stated (Journal of Surgical Oncology, April 1, 2005, Vol. 90:1, pp. 1-9).
However, Whitworth and Rewcastle stressed that large trials using cryoablation in fibroadenomas were necessary to judge the long-term results. Other groups agreed. In a 2006 technology review, the California Technology Assessment Forum (CTAF) declared that because "there are no comparative studies in the literature … it is unclear if cryoablation is being advocated as an alternative to observation or excisional biopsy" (CTAF, February 15, 2006).
Littrup said he believes that cryotherapy trumps other ablation procedures in any organ including the breasts. The advantages are "excellent visualization by US, CT, and/or MRI, excellent healing (about 80% to 90% volume reduction by six to 12 months), (and) minimal procedure pain done on outpatient basis," he explained. "So other than cardiac ablation for arrhythmias, breast ablation for all masses should be one of the ideal areas for cryo."
Cryo costs
There are currently two players in the field of cryoprobes for fibroadenoma treatment: Sanarus of Pleasanton, CA (Visica Treatment System) and Galil Medical of Yokneam, Israel (SeedNet systems). Sanarus markets its system in the U.S. (and funded the FACT registry). Galil Medical is currently conducting a postmarket study in the U.S. on using cryoablation for fibroadenoma treatment, according to a Galil spokesperson.
The current cost for breast cryotherapy averages about $3,000, which is about half the cost of surgery, noted Holms. Reimbursement is available but still uneven. According to a recent ACR Bulletin, a new Centers for Medicare and Medicaid Services (CMS) code was released in 2007, stating that ultrasound guidance was an integral part of the cryoablation procedure and should not be reported separately (CPT 0120T replaced with CPT 19105).
But some private insurers continue to regard cryoablation for fibroadenoma as experimental, and cited the limited number of controlled clinical trials as insufficient to prove its efficacy.
Others, such as UnitedHealthcare, will reimburse for the procedure when the recommendations of the American Society of Breast Surgeons (ASBrS) are followed. The ASBrS specifies that for cryoablation eligibility, the lesion must be sonographically visible, the lesion should be less than 3 cm in diameter, and that diagnosis has to be histologically confirmed.
The ASBrS is currently not sponsoring any clinical trials on this technique in cryoablation or working to change reimbursement, said Whitworth, who chairs both the board of directors and the research committee and board of directors of the ASBrS.
Ultimately, physicians and the women themselves will have to lead the charge to make cryoablation one of the go-to treatment methods for fibroadenomas.
"This will be primarily driven by patient preference. Like most other treatments, it can also be driven by physician preference," explained Whitworth, who is also a clinical associate professor of surgery at Vanderbilt University in Nashville. "Physician preference can be affected by the ability of the physician to provide the treatment. Percutaneous excisional biopsy of palpable fibroadenomas came along as cryoablation technology was moving forward. Most physicians who are able to do both favor percutaneous excision for a patient with no particular preference. Some patients desire (not to have) tissue … removed and so prefer cryoablation."
By Kathlyn Stone
AuntMinnie.com contributing writer
October 26, 2007
Additional reporting by staff writer Shalmali Pal.
Related Reading
Ultrasound follow-up acceptable for benign breast masses that appear benign, June 26, 2007
Cryoablation reduces metastatic cancer pain, November 27, 2006
Copyright © 2007 AuntMinnie.com