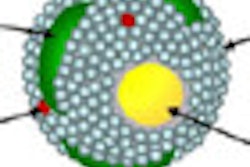
Ultrasmall gadolinium oxide (US-Gd2O3) nanoparticles provide strong positive contrast enhancement at clinical magnetic field strengths. For this reason, they are being developed as positive contrast agents for molecular and cellular MRI.
Recent studies have demonstrated that these particles can be used to label and track cells in vivo. Positive contrast agents enhance the signal, which results in a local "brightening" in MRI scans. These agents could represent an attractive tool for examining cell diffusion processes.
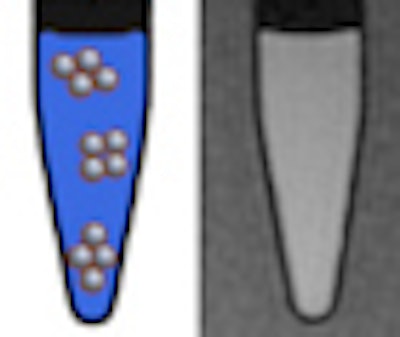
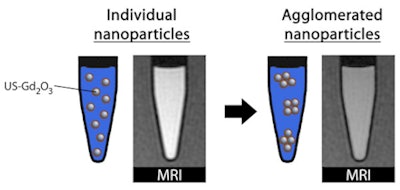
However, once internalized into cells, nanoparticles tend to agglomerate in cell endosomes. But how much aggregation takes place? And how does this affect the performance of Gd2O3 nanoparticles in MRI cell-tracking studies?
To answer these questions, Canadian and Belgian researchers have conducted a series of investigations. The researchers, directed by Marc-André Fortin, PhD, from the department of engineering materials at Université Laval, reported earlier this year on the use of US-Gd2O3 particles for "positively contrasted" cellular imaging. They were joined for this work by Yves Gossuin, PhD, from Université de Mons (Nanotechnology, July 2011, Vol. 22:29).
Gadolinium is a paramagnetic element widely used in MRI. However, most gadolinium complexes used nowadays are not suitable for molecular and cellular imaging because of the relatively weak unitary impact of each one of the gadolinium atoms on the relaxation time of hydrogen protons (providing the "signal" detected in MRI).
To enhance the detectability of gadolinium-based contrast agents, one strategy is to bring hundreds of gadolinium atoms into so-called "ultrasmall nanoparticles." Adequately coated, such particles are very efficiently ingested by cells and are considered as promising "probes" for cell tracking in MRI.
 |
| The study establishes the potential of ultrasmall Gd2O3 nanoparticles as a "positive" contrast agent for MRI that is particularly efficient and potentially very useful at clinical magnetic field strengths (1.0-3.0 tesla). |
Field strength assessment
The team recently measured the efficiency of Gd2O3 suspensions for MRI applications (longitudinal and transverse relaxivities). Data acquired at magnetic field strengths typical of most hospital scanners (0.5-3.0 tesla) were compared with measurements at higher magnetic field strengths (7.0-11.7 tesla), as found in MRI systems mainly used in preclinical research.
At every magnetic field, the longitudinal relaxivity (r1) decreased upon agglomeration, while remaining high enough to provide positive contrast. The transverse relaxivity (r2) decreased slightly at 0.47 and 1.41 tesla, which is good for clinical MRI; however, it was enhanced at higher fields (7.0 and 11.7 tesla) upon agglomeration, thereby affecting the positive contrast enhancement effect. This is due to the total magnetic moment developed during agglomeration, since paramagnetic aggregates are considered as large magnetized spheres.
Given that agglomeration is susceptible to occur once particles are ingested in the cells, this is a significant finding to clearly establish the magnetic field strengths at which US-Gd2O3 particles might be preferentially used.
Hydrated coatings
The researchers suggest the development of highly hydrated nanoparticle coatings to maintain the interaction of surface gadolinium with hydrogen-1 (H-1) protons in spite of aggregation. Modulating the thickness of the nanoparticle polymer coating would also help to prevent the decrease of transversal relaxation time at high magnetic fields, which is caused by the close proximity of Gd2O3 nanocrystals upon agglomeration.
The study clearly establishes the potential of ultrasmall Gd2O3 nanoparticles as a "positive" contrast agent for MRI that is particularly efficient and potentially very useful at clinical magnetic field strengths (1.0-3.0 tesla).
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.