NorthStar Medical Radioisotopes parent NorthStar Medical Technologies has signed an agreement with imaging agent developer Capella Imaging to exclusively license an investigational cardiac SPECT radiopharmaceutical.
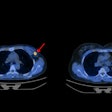
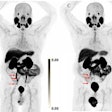
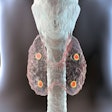
Called FibroScint (Tc-99m F4A), the radiopharmaceutical is a fibrin-targeted peptide compound labeled with technetium-99m (Tc-99m). NorthStar said that FibroScint could have potential applications in SPECT imaging for cardiovascular conditions such as thrombus associated with left ventricular assist devices (LVADs), deep vein thrombosis, pulmonary embolism, and acute coronary syndrome.
The initial clinical investigation of FibroScint will be an exploratory phase I study that will assess its potential use in identifying thrombus in patients with serious heart failure who use an LVAD, according to NorthStar. The firm said it anticipates that FibroScint's first indication will receive an orphan drug designation by the U.S. Food and Drug Administration.
NorthStar noted that FibroScint complements its RadioGenix isotope separation system; Tc-99m produced by the RadioGenix system will be used in the planned clinical studies of FibroScint. The company will also collaborate with Capella to further develop the radiopharmaceutical.
Under the deal, NorthStar will invest $1 million in Capella equity in conjunction with Capella seeking federal funding grants. Both sources of funds will be used for the completion of phase I clinical development through an exploratory investigational new drug application. In addition, NorthStar has the option to make additional equity investments to support future clinical trials.
NorthStar will also send additional success payments to Capella, contingent on the company completing certain regulatory and revenue milestones. Royalties will also be paid upon the commercialization of FibroScint in each of three potential diagnostic indications, according to NorthStar.