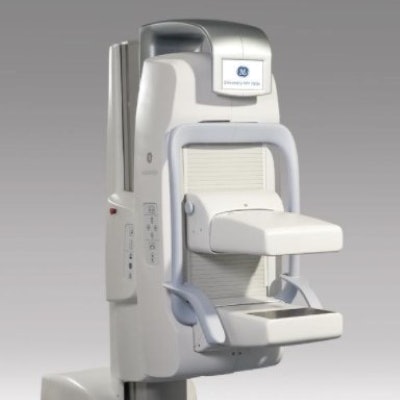
Breast cancer screening firm SmartBreast has acquired assets related to the Discovery NM750b molecular breast imaging (MBI) business of GE Healthcare, including scanner designs, patents, and service contracts for GE's MBI installed base.
SmartBreast plans to sell the rebranded system as Eve Clear Scan e750, and it will support the existing installations of the system at scores of sites around the world.
The deal is part of SmartBreast's plan to become the leading provider of MBI technology. The company earlier this year announced its acquisition of Dilon Diagnostics, another MBI manufacturer. That agreement included rights to Dilon's Dilon 6800 scanner, as well as patents and other technology. With both deals combined, SmartBreast has an installed base of 217 MBI systems worldwide.
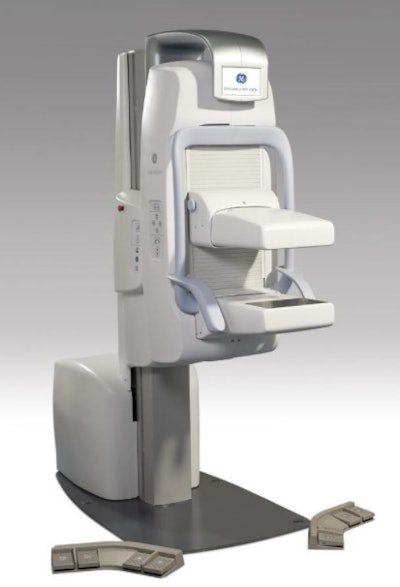 SmartBreast will sell the MBI system as Eve Clear Scan e750. Image courtesy of SmartBreast.
SmartBreast will sell the MBI system as Eve Clear Scan e750. Image courtesy of SmartBreast.GE first introduced Discovery NM750b at the RSNA 2010 meeting, positioning the system as an adjunct to mammography rather than a screening tool. In 2016, GE signed a deal with Dilon Diagnostics to make that company the exclusive distributor of Discovery NM750b in North America.
A major distinguishing feature of Discovery NM750b is its cadmium zinc telluride (CZT) digital detectors, which GE said it will continue to supply to SmartBreast after the sale.
MBI can provide a secondary imaging technique after an inconclusive primary examination with conventional mammography. In particular, the technology can be useful for women with dense breast tissue, which can cause difficulties for conventional x-ray-based imaging techniques.
As a sign of MBI's utility, SmartBrief highlights a 2015 study in which researchers reported that mammography found 3.2 cancers per 1,000 women with dense breasts; adding MBI increased the yield of cancers discovered to 12 per 1,000.