The U.S. Food and Drug Administration (FDA) has accepted a new drug application (NDA) from Blue Earth Diagnostics for its investigational radiohybrid prostate-specific membrane antigen (PSMA) F-18 rhPSMA-7.3 radiopharmaceutical.
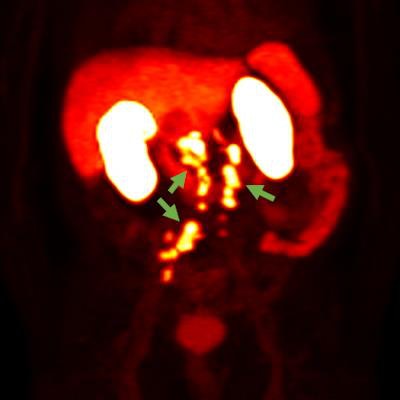
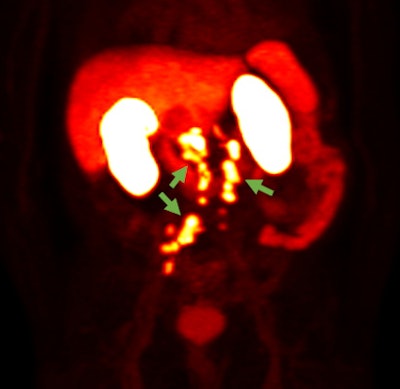 F-18 rhPSMA-7.3 PET image showing prostate cancer spread beyond the prostate region. Photo courtesy of Blue Earth Diagnostics.
F-18 rhPSMA-7.3 PET image showing prostate cancer spread beyond the prostate region. Photo courtesy of Blue Earth Diagnostics.Blue Earth submitted the NDA for the use of F-18 rhPSMA-7.3 in PET imaging of prostate cancer, according to the vendor. The submission was supported by clinical data from one prospective phase I study and two prospective phase III clinical trials, Blue Earth said.