The Society of Nuclear Medicine and Molecular Imaging (SNMMI) has highlighted a PET study in women with breast cancer featuring a radiotracer that could replace invasive biopsies for detecting metastatic disease.
In a phase II clinical trial, a group led by Ali Alhuseinalkhudhur, MD, of Uppsala University in Uppsala, Sweden, tested whether a tracer named gallium-68 (Ga-68) ABY-025 could detect metastatic breast cancer in patients with aggressive human epidermal growth factor receptor 2 (HER2)-positive cases.
"While HER2 status can be confirmed by biopsy results in early-stage breast cancer, it is more complicated in advanced disease where multiple metastases might have a heterogeneous HER2 expression," Alhuseinalkhudhur said, in a SNMMI release. The study was published in the September issue of the Journal of Nuclear Medicine.
Up to 20% of breast cancer patients have tumors that overexpress HER2 cellular receptors, yet the expression of these proteins may vary within the same patient and over time. Thus, biopsies are limited in detecting disease in these cases, and subsequent HER2-targeted treatment failure is common, Alhuseinalkhudhur explained.
In this study, the researchers investigated whether Ga-68 ABY-025 PET/CT – a tracer that binds to HER2 – could serve as a noninvasive tool for quantifying whole-body HER2 expression and ultimately improve treatment planning.
The group recruited 40 patients with known positive HER2 status: 19 with primary breast cancer and 21 with metastatic breast cancer. Patients underwent whole-body Ga-68 ABY-025 PET/CT scans as well as F-18 FDG PET/CT scans and biopsies at baseline.
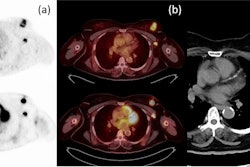
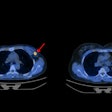
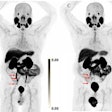
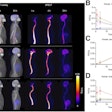
 Ga-68 ABY-025 PET/CT and F-18 FDG-PET/CT images at baseline with F-18 FDG PET/CT follow-up after two cycles of treatment in biopsy-confirmed HER2-positive disease. (A) Patient with high Ga-68 ABY-025 uptake (SUVmax, 21), who previously received three lines of treatment. F-18 FDG-PET/CT follow-up showed complete metabolic response. (B) Patient with low uptake (SUVmax, 5.4), who previously received three lines of treatment. F-18 FDG PET/CT follow-up showed disease progression (a 68% increase in tumor glycolysis) despite HER2-targeted treatment. (C) Patient with heterogeneous Ga-68 ABY-025 uptake, who previously received seven lines of treatment. F-18 FDG-PET/CT follow-up showed heterogeneous response, with lesions higher in Ga-68 ABY-025 uptake tending to have better response. Arrows indicate biopsy sites. Image courtesy of the Journal of Nuclear Medicine.
Ga-68 ABY-025 PET/CT and F-18 FDG-PET/CT images at baseline with F-18 FDG PET/CT follow-up after two cycles of treatment in biopsy-confirmed HER2-positive disease. (A) Patient with high Ga-68 ABY-025 uptake (SUVmax, 21), who previously received three lines of treatment. F-18 FDG-PET/CT follow-up showed complete metabolic response. (B) Patient with low uptake (SUVmax, 5.4), who previously received three lines of treatment. F-18 FDG PET/CT follow-up showed disease progression (a 68% increase in tumor glycolysis) despite HER2-targeted treatment. (C) Patient with heterogeneous Ga-68 ABY-025 uptake, who previously received seven lines of treatment. F-18 FDG-PET/CT follow-up showed heterogeneous response, with lesions higher in Ga-68 ABY-025 uptake tending to have better response. Arrows indicate biopsy sites. Image courtesy of the Journal of Nuclear Medicine.
After two cycles of treatment, patients underwent F-18 FDG PET/CT scans again to establish changes in tumor glycolysis – essentially, a broad measurement of how much glucose tumor cells use to fuel the growth of tumors.
Then, the researchers compared standardized uptake values (SUV) of the Ga-68 ABY-025 radiotracer in the target tumors with the biopsy results and FDG-PET/CT changes in tumor glycolysis before and after treatment.
According to the findings, Ga-68 ABY-025 PET/CT successfully enabled quantification of HER2 expression, and baseline uptake levels of the radiotracer correlated significantly with tumor responses observed on the FDG-PET scans, particularly in patients with metastatic disease.
Importantly, however, there was no significant association between Ga-68 ABY-025 uptake with biopsy-derived HER2 status. This means that biopsies may not always reflect the "biologic availability" of the HER2 receptors, the researchers suggested.
“The ability of Ga-68 ABY-025 PET/CT to provide a whole-body visualization of HER2 expression and to predict metabolic response is advantageous and exceeded the biopsy-based approach for metastatic breast cancer patients," Alhuseinalkhudhur said.
Ultimately, by providing whole-body quantification of HER2 expression, Ga-68 ABY-025 PET/CT could play a valuable role in treatment planning, the researchers suggested.
"Limitations in the metastatic setting still hinder accurate biopsy-based evaluation of heterogeneous HER2 expression. Hence, the advantage of noninvasive techniques such as Ga-68 ABY-025 PET/CT prevails," Alhuseinalkhudhur and colleagues concluded.
A link to the full article can be found here.