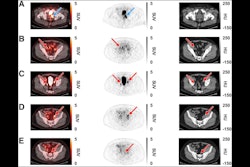
Archeus Technologies has received U.S. Food and Drug Administration (FDA) clearance of its Investigational New Drug (IND) application for its ART-101 small molecule for prostate cancer imaging and treatment.
ART-101 targets prostate-specific membrane antigen (PSMA) and is similar to other FDA-approved imaging and therapeutic agents, except that ART-101 has exhibited higher tumor uptake and retention, as well as lower normal tissue and salivary gland uptake, according to Archeus.
IND clearance enables the company to initiate a phase I clinical trial in men with metastatic castration-resistant prostate cancer (mCRPC). That trial is expected to begin later this year, the company said.