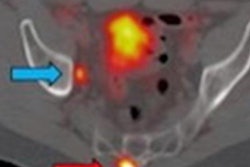
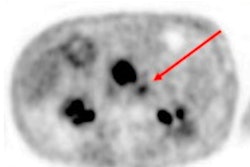
The U.S. Food and Drug Administration (FDA) has approved the first PET radiotracer targeting lesions positive for prostate-specific membrane antigen (PSMA) in men with prostate cancer, a development the agency believes has important implications for patient care.
Approval for the intravenously delivered radiopharmaceutical -- gallium-68 (Ga-68) PSMA-11 -- was granted to the University of California, San Francisco (UCSF) and the University of California, Los Angeles (UCLA). It is indicated for prostate cancer patients with suspected metastases and who have a good prognosis for surgery or radiation therapy as well as those who have elevated prostate-specific antigen (PSA) levels suggestive of recurrence.
The FDA's approval was supported by two prospective clinical trials that enrolled a total of 960 men with prostate cancer. In one study, Ga-68 PSMA-11 with PET/CT or PET/MRI was helpful for identifying those with metastases who were good candidates for surgical treatments.
"The availability of this information prior to treatment is expected to have important implications for patient care," the agency noted in a statement about the approval. "For example, it may spare certain patients from undergoing unnecessary surgery."
In the second study, the new radiopharmaceutical was helpful for the detection of recurrent disease in men with rising PSA, which the agency said is "important information that may impact the approach to therapy."
In a joint statement, UCLA and UCSF noted that it is "rare for academic institutions to obtain FDA approval of a drug." PSMA-PET has outperformed fluciclovine-PET, which has been the standard of care, and should be "strongly considered both before initial treatment in men with high-risk cancers and in cases of cancer recurrence after surgery or radiation to provide more precise care," researchers advised.
Under the current approval, PSMA-PET may only be offered at UCLA and UCSF, but hospitals may apply for expedited approval following the FDA's clearance.