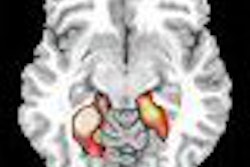
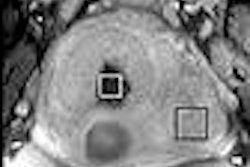
GE Healthcare said that the Danish Medicines Agency has approved its Omniscan MR contrast agent for use in myocardial perfusion imaging and delayed enhancement for detecting coronary heart disease.
The specific approval to use an MR contrast agent for myocardial perfusion imaging is the first for GE in the world. Omniscan does not have approval for this indication in the U.S., according to the Chalfont St. Giles, U.K.-based vendor.
By AuntMinnie.com staff writers
September 30, 2005
Related Reading
GE launches $1.2 billion bid for IDX, September 29, 2005
GE adds Centricity order, September 7, 2005
GE names Riesenberg to IT post, August 18, 2005
GE, Insightec ink new deal, August 17, 2005
GE adds to VHA contract, August 5, 2005
Copyright © 2005 AuntMinnie.com