MRI contrast developer Epix Pharmaceuticals of Lexington, MA, has released details from a phase IIa open-label clinical trial of its EP-2104R fibrin-binding imaging agent.
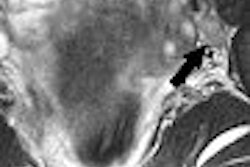
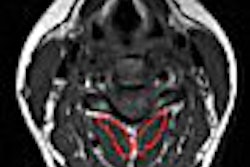
The 52-patient trial found that the agent was well-tolerated and was able to enhance blood clots that were seen on the MRI images and detect those previously unseen in all six body systems studied, company said.
In many cases, current modalities are unable to provide a definitive thrombus diagnosis and multiple tests are needed for a diagnosis, according to Epix. Using EP-2104R could potentially reduce the number of tests, resulting in significant cost savings, the company said.
With the positive trial data, Epix intends to seek a collaboration to continue to EP-2104R development.
By AuntMinnie.com staff writers
November 30, 2006
Related Reading
Epix nets Canadian OK for Vasovist, November 6, 2006
Epix nets Australian approval, September 20, 2006
Epix regains Nasdaq symbol, September 15, 2006
FDA rejects Epix appeal, August 28, 2006
Epix, Predix complete merger, August 17, 2006
Copyright © 2006 AuntMinnie.com


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











