Device manufacturer Medtronic said the U.S. Food and Drug Administration (FDA) has approved an investigational device exemption (IDE) application for a study of the firm's MRI-guided laser ablation technology in a potential new indication.
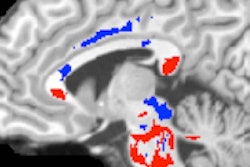
The Stereotactic Laser Ablation for Temporal Lobe Epilepsy (SLATE) study will evaluate the use of Medtronic's Visualase MRI-guided laser ablation technology for epilepsy treatment in patients with drug-resistant mesial temporal lobe epilepsy (MTLE). Medtronic hopes the study will lead to an expanded indication for Visualase to treat MTLE, the most common form of partial or localization-related epilepsy.
The study will include about 120 adults with drug-resistant MTLE who will be treated at centers across the U.S. Patients will be followed for a year after the Visualase procedure to assess for freedom from seizures, adverse events, quality of life, and neuropsychological outcomes, the company said.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











