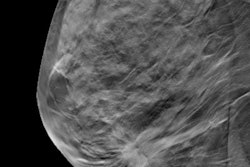
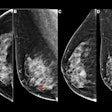
The design of the Tomosynthesis Mammographic Imaging Screening Trial (TMIST) may be less favorable toward digital breast tomosynthesis (DBT) and may have unintended consequences for women's health, according to Dr. Daniel Kopans of Harvard University in an analysis published June 24 in Clinical Imaging.
 Dr. Daniel Kopans.
Dr. Daniel Kopans.Kopans said that while DBT will "almost certainly" become the standard of care in breast imaging, the design of the TMIST study may provide misleading information and the trial may be underpowered to show a benefit for the technology.
"Instead of using a reduction in deaths as the endpoint, benefit through TMIST is predicated on a reduction in advanced cancers in the DBT group. This is a questionable 'endpoint' [since] a reduction in advanced cancers is not necessary as proof of benefit," he said. "In addition, the trial may be underpowered so that even if DBT shows a benefit it may not be able to achieve statistical significance."
TMIST is a randomized breast screening trial designed to help researchers learn about the best ways to find breast cancer in women who have no symptoms. It compares standard 2D digital mammography with digital breast tomosynthesis, which creates 3D images from different 2D acquisitions of the breast.
DBT has shown promise in reducing recall rates and showing lower interval cancer rates for women. However, it isn't definite whether or not DBT is better than standard 2D mammography at finding breast cancers before they become more difficult to treat.
Women ages 45 to 74 who enter the TMIST trial are randomly assigned to either 2D or 3D mammography. Researchers are looking to determine if the rate of advanced breast cancers will be reduced among volunteers undergoing DBT versus digital mammography.
However, if there is no statistically significant difference between the two, screening using DBT will not be deemed superior to 2D mammography.
Kopans wrote that since DBT cannot have any effect on advanced cancers in the year they are already there, data from the first year of screening -- when prevalence cancers are "likely the largest number" -- will be unusable. And if they are used, they will, "inappropriately" dilute the results.
"At the least, it needs to be determined if the statistical power will be lost because the rate of advanced cancers in the prevalence screen cannot be used," he said. "This may mean that even with the reduced power requirement, TMIST will not have the statistical power to answer its primary question. If this is not recognized, its results may be grossly misleading."
Kopans also said that TMIST may unintentionally reduce access to mammography screening by not including women ages 40 to 44. Societies such as the American Cancer Society and the American College of Radiology support mammography screenings in women starting at age 40.
Kopans also said that TMIST's protocol of screening postmenopausal women every two years instead of annually may reduce screening access further.
Another potential design issue Kopans pointed out was detection of ductal carcinoma in situ (DCIS), saying that while DBT detects more invasive cancers than 2D mammography, it does not increase the detection rate of DCIS.
"It could be argued that the imaging part of TMIST is a waste of valuable resources," Kopans wrote. "By the time TMIST is complete, it is almost certain that all FFDM systems will have been replaced by DBT devices in the U.S."
He also added that since TMIST does not have a mortality endpoint, it will only investigate whether DBT results in a decline in advanced cancers, "ignoring the fact that many women still die from cancers that are not advanced at the time of diagnosis."