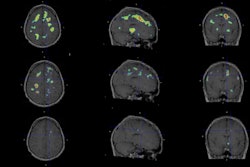
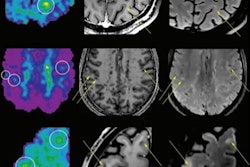
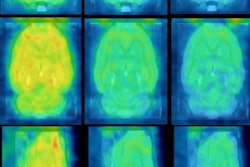
The U.S. Food and Drug Administration (FDA) has approved human trials for a new PET radiotracer that could help diagnose multiple sclerosis (MS) in its early stages.
Myeliviz, developed by researchers at Case Western Reserve University, is designed to target and bind to the sheathing -- known as myelin -- that surrounds the nerves and is affected by MS. The tracers binding to myelin then can be visualized on PET images. By doing so, Myeliviz might provide evidence to help physicians determine the presence and degree of damage to patients' central nervous systems from MS.
The tracer will be tested in clinical trials involving healthy volunteers at Cleveland Clinic Mellen Center for Multiple Sclerosis. The first human trials for Myeliviz are funded through a $1.7 million grant from the U.S. National Institutes of Health.