Research on prostate cancer imaging agents that can be produced faster and cheaper than current radiotracers -- and also have therapeutic potential -- was presented by a group from Memorial Sloan Kettering Cancer Center at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2020 virtual annual meeting.
The new radiopharmaceuticals, 124I-MSK-PSMA1 and 131I-MSK-PSMA1, target prostate-specific membrane antigens (PSMA), which tend to highly overexpress in patients with primary and metastatic prostate cancer.
Existing PSMA-targeting agents require multiple production steps that reduce their yield and make it significantly more difficult to produce therapeutic doses, the researchers noted. But the new agents utilize an easily available chemical precursor to produce larger yields without the need for expensive, high-pressure liquid chromatography machinery.
"Our goal was to develop a radiopharmaceutical that can be produced very easily in high yields and high purity without requiring specialized equipment," stated senior author and radiochemist Naga Vara Kishore Pillarsetty, PhD, in a press release.
Pillarsetty and his team conducted a series of preclinical studies to demonstrate the efficacy of the new agents for prostate cancer diagnosis and treatment.
In an in vitro study, they performed saturation-binding assays in prostate cancer cells that they later harvested and used to count radioactivity. In several in vivo studies, they performed PET imaging and biodistribution on mice with prostate cancer xenografts.
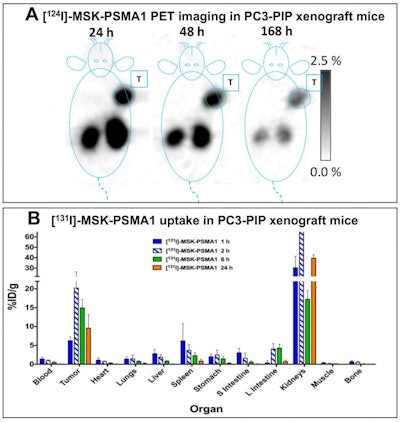 Image A shows PET images (coronal slices) of 124I-MSK-PSMA1 in mice bearing prostate cancer cell xenografts on the right shoulder. Image B shows ex vivo biodistribution data of 131I-MSK-PSMA1 in mice bearing prostate cancer cell xenografts. Image courtesy of Kalidindi TM, et al., Pillarsetty Lab, MSKCC, New York, NY.
Image A shows PET images (coronal slices) of 124I-MSK-PSMA1 in mice bearing prostate cancer cell xenografts on the right shoulder. Image B shows ex vivo biodistribution data of 131I-MSK-PSMA1 in mice bearing prostate cancer cell xenografts. Image courtesy of Kalidindi TM, et al., Pillarsetty Lab, MSKCC, New York, NY.The results showed that both the 121I and 131I versions of MSK-PSMA1 generated high yields without producing volatile byproducts, the researchers noted. The studies also demonstrated high specificity, favorable pharmacokinetics, and rapid clearance from nontarget tissue in prostate cancer cell and tumor xenograft models.
"The exceptional tumor targeting and clearance from nonPSMA-expressing tissues make 124I-MSK-PSMA1 an excellent PET imaging agent and the corresponding 131I-MSK-PSMA1 a highly potent radiotherapeutic agent," stated lead author and senior research technician Teja Muralidhar Kalidindi.
The Memorial Sloan Kettering team is now working with nuclear medicine clinicians to help translate their findings into an in-person clinical trial for the two versions of MSK-PSMA1. They also plan to develop a kit formulation to facilitate production of the agents for the global research community.
"We believe that 124/131I-MSK-PSMA1 has the potential to become part of the armamentarium available to the nuclear medicine and molecular imaging community for the diagnosis and treatment of metastatic castration-resistant prostate cancer patients," Kalidindi stated.