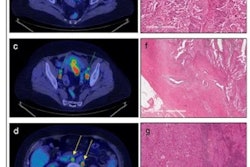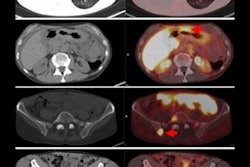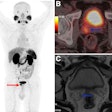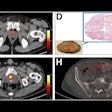
PET imaging with the radiotracer F-18 fluoroestradiol (FES) can help determine how well patients will fare after a diagnosis of endometrial cancer, according to a study published October 2 in the Journal of Nuclear Medicine.
Japanese researchers not only found that standardized uptake values (SUV) from FES-PET scans of the primary tumor were independent prognostic factors for progression-free survival but also were "significantly associated" with chances for overall survival.
"These data suggest that pretreatment F-18 FES-PET might be useful in determining therapeutic strategies and could improve the prognosis for patients with endometrial cancer," wrote the authors, led by Dr. Shizuka Yamada from University of Fukui.
The current challenge regarding the evaluation of endometrial cancer cases is that there currently are no biomarkers to accurately gauge the prognoses for patients before they undergo surgery, which some women delay in order to preserve their fertility. While previous studies have suggested that a high level of estrogen receptor α (ERα) bodes well for patient survival, invasive surgery or biopsy is required to gather a tissue sample to assess ERα.
So how might FES-PET help in these situations? The tracer could be quite influential based on a 2011 study by the current authors that linked FES uptake with levels of ERα in endometrial cancer patients. Those findings followed their 2009 study that showed mean SUV on FES-PET combined with mean SUV from FDG-PET "could indicate tumor aggressiveness in patients with endometrial cancer," Yamada and colleagues wrote.
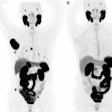
Fast forward to this prospective study, in which 67 patients (median age 59.1; range, 32-81) with untreated endometrial cancer ranging from stage IA to IVB were enrolled between December 2004 and December 2015. All subjects underwent FES-PET (Advance, GE Healthcare) and FDG-PET/CT (Discovery LS, GE) on two separate days within one week and before cancer treatment, which included surgery and follow-up assessment of surgical specimens.
The primary endpoints were progression-free survival and overall survival, with a median follow-up period of 60 months (range, 10.4-60 months). During that time, 14 patients (21%) showed tumor progression and six patients (9%) died.
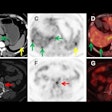
After performing statistical analysis of the receiver-operating characteristic (ROC) curve to determine SUV cutoffs, the calculations indicated that the lower FES SUV cutoff (2.63) performed significantly better at determining tumor-free progression and overall survival with area under the curve (AUC) of 0.813 and 0.79, respectively. By comparison, the higher FDG SUV cutoff (8.28) achieved AUCs of only 0.557 and 0.635, respectively.
| FES, FDG, FES-FDG SUVs for predicting patient outcomes | |||
| FES | FDG | FES-FDG | |
| Tumor-free progression | |||
| Sensitivity | 78.6% | 61.5% | 76.9% |
| Specificity | 86.6% | 54% | 72% |
| Overall survival | |||
| Sensitivity | 83.3% | 83.3% | 83.3% |
| Specificity | 78.7% | 54.4% | 87.7% |
In addition, an FES SUVmean of 3.15 in the primary tumor also indicated the presence of lymphovascular space involvement and was significantly more effective than an FDG SUVmean of 10.40 to indicate the anomaly (p < 0.001).
"These findings suggest that F-18 FES-PET could be used to determine therapeutic strategies such as adjuvant chemotherapy and lymphadenectomy, and thus potentially improve the prognosis of patients with endometrial cancer," Yamada and colleagues concluded.
They also recommended additional, larger studies to investigate how menopausal status and treatment after endometrial cancer recurrence to evaluate the predictive prowess of FES-PET.