Imaging software developer Syntermed highlighted that it will be the first distributor in the U.S. of Pylarify AI, Lantheus Medical Imaging's artificial intelligence (AI) program for assisting standardized quantification of prostate-specific membrane antigen (PSMA)-PET/CT scans.
Lantheus received approval from the U.S. Food and Drug Administration (FDA) in May 2021 for Pylarify (F-18 DCFPyL), a PET radiotracer that targets PSMA, with clearance for Pylarify AI following in July.
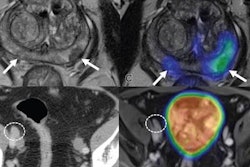
Pylarify AI uses a deep-learning algorithm that has been trained and validated on more than 3,000 images. It allows healthcare professionals and researchers to perform standardized quantitative assessment of PSMA-PET/CT images.
PSMA is a protein expressed on prostate cancer cells. Imaging and quantifying PSMA in patients with suspected prostate cancer can help clinicians determine effective treatment approaches.
The Pylarify AI software can be used as a secure web cloud application or within the firewall of an institution on a local server, the company said. After it is deployed, the application can be integrated into an institution's existing clinical workflow.