Blue Earth Diagnostics is highlighting results from a phase III clinical trial that tested its diagnostic PET imaging radiotracer F-18 rhPSMA-7.3 in newly diagnosed prostate cancer patients.
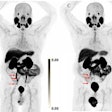
In a study presented on February 16 at the 2023 American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium in San Francisco, researchers analyzed detection rates for F-18 rhPSMA-7.3 PET in patients with distant metastatic tumors. Out of 335 men included in the analysis, 10%-15% (35-49) had verified metastatic lesions.
By region, lesions verified by F-18 rhPSMA-7.3 PET imaging were most common in bone, ranging from 6% to 11% across readers. Similar data were shown among a subgroup of patients who had negative baseline conventional imaging, the researchers reported.
F-18 rhPSMA-7.3 is a radiohybrid agent consisting of a prostate-specific membrane antigen ligand that may be combined with isotopes such as F-18 for imaging purposes or Lutetium-177 or Actinium-225 for therapeutic use. It is currently under review by the U.S. Food and Drug Administration (FDA). Blue Earth has included these latest results in its new drug application.