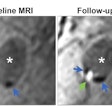
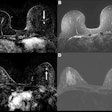
Siemens Healthcare has received U.S. Food and Drug Administration (FDA) clearance for a new surface coil for MR prostate imaging.
Running in tandem with Siemens' syngo MR E11 software architecture, the new SEEit surface coil enables users of Siemens' Magnetom Aera 1.5-tesla and Magnetom Skyra 3-tesla MRI systems to perform routine prostate exams in 10 minutes without need of an endorectal coil, which can cause patient discomfort, Siemens said.
Siemens' Direct RF and high-density Tim 4G coil technology, coupled with Siemens' Resolve diffusion technology, provide the necessary signal-to-noise ratio and resolution for users to perform the exams using only the company's Body 30/60 surface coil.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)