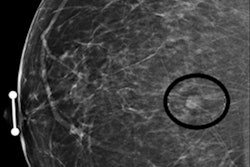
Image-guided biopsies performed after breast cancer patients have been treated with neoadjuvant chemotherapy can predict the presence of residual cancer, potentially avoiding the need for surgery, a multinational team of researchers reported in an article published online October 7 in JAMA Surgery.
In a study involving 166 women from three institutions, the researchers found that a standard image-guided, vacuum-assisted biopsy to gather six or more representative samples of a tumor bed that's 2 cm or smaller could either find residual cancer or predict complete pathologic response with a less than 5% false-negative rate.
"Postneoadjuvant chemotherapy image-guided biopsy may identify pathologic complete response in the breast in selected patients; these findings can inform the design of de-escalation trials testing the elimination of surgery in exceptional responders," wrote the researchers led by first author Dr. Marios Konstantinos Tasoulis, PhD, of the Royal Marsden NHS Foundation Trust in London, U.K., and senior author Dr. Henry Kuerer, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
Image-guided biopsy of the tumor bed or residual abnormalities found on imaging after neoadjuvant chemotherapy is gaining popularity as a predictive tool for assessing residual cancer. The technique can facilitate risk-adaptive surgery, and it can potentially even identify exceptional treatment responders for de-escalation of local therapy, according to the researchers. Published studies in the literature have also been promising.
To further assess the accuracy of postchemotherapy breast biopsy for predicting residual cancer, researchers analyzed 166 patients who had received image-guided biopsy prior to surgery at three institutions: Royal Marsden Hospital, MD Anderson Cancer Center in Houston, TX, and Seoul National University Hospital in Seoul, South Korea. Diagnostic accuracy was assessed using final surgical pathology results as the reference standard.
Of the 166 women, 143 received image-guided vacuum-assisted biopsy and 23 had core-cut biopsies. Ultrasound guidance was used in 129 (77.7%) patients, while stereotactic guidance was utilized in 37 (22.3%) patients.
The biopsy needle gauge range was 7-14, with a median of 10. The median number of biopsies samples was 6, with a range of 2-18.
In the 159 patients who had a representative image-guided biopsy, the false-negative rate was 18.7%. However, the best results were observed in a subgroup analysis of 76 patients who had a complete or partial clinical treatment response and a residual imaging abnormality of 2 cm or smaller with at least six vacuum-assisted biopsy samples.
| Performance of postchemotherapy image-guided biopsy in predicting residual cancer or complete treatment response | ||
| Overall patient group | Patients with complete or partial clinical response and a residual imaging abnormality of 2 cm or smaller with at least 6 vacuum-assisted biopsy samples | |
| False-negative rate | 18.7% | 3.2% |
| Negative predictive value | 84.3% | 97.4% |
| Overall accuracy | 85.5% | 89.5% |
"This large multicenter pooled data analysis suggests that a standardized protocol using image-guided [vacuum-assisted biopsy] of a tumor bed measuring 2 cm or smaller with 6 or more representative samples allows reliable prediction of residual disease," the authors wrote.