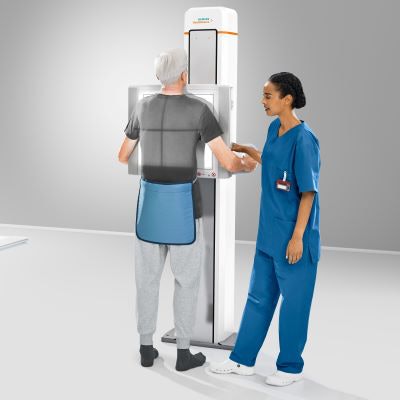
Siemens Healthineers has garnered U.S. Food and Drug Administration (FDA) clearance for Ysio X.pree, the firm's new premium digital radiography (DR) system.
First announced in June, the ceiling-mounted system features the vendor's MyExam Companion user interface. Designed to proactively provide guidance to technologists, MyExam Companion uses artificial intelligence (AI) to identify optimal acquisition and reconstruction parameters for each patient, Siemens said.
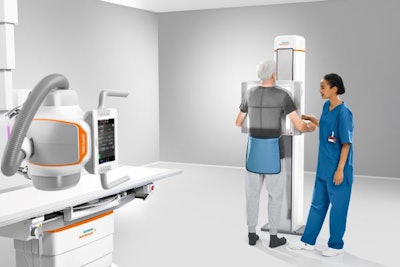 The Ysio X.pree DR system includes automation tools to improve efficiency. Image courtesy of Siemens Healthineers.
The Ysio X.pree DR system includes automation tools to improve efficiency. Image courtesy of Siemens Healthineers.Ysio X.pree also includes MyExam 3D Camera, which gives users a live view of the x-ray room and enables patient positioning and advanced collimation from the control room workstation. Siemens said it has also incorporated a new image processing database that provides "intelligent" dynamic range optimization and noise elimination, as well as gridless acquisition and AI-based image cropping.