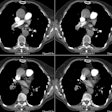
Pulmonary Embolism:
View cases of pulmonary embolism
Clinical:
Pulmonary embolism (PE) is a frequently overlooked diagnosis
which can be associated with significant mortality if untreated
(mortality previously approached 20-35%) [134]. More than 80% of
deaths from PE occur in the first 30 minutes and 90% within the
first 2.5 hours of the event [134]. However, previous mortality
estimates were derived from inpatient data and more current risk
estimates based on untreated ot missed PE in ambulatory patients
reveal mortality and recurrence rates of less than 5% [168,169].
PE should be thought of as a spectrum of disease with outcomes
varying from inconsequential to deadly depending on other
comorbid factors [168]. There is now abundant evidence that PE
is being overdiagnosed and that some of the cases involve
clinically insignificant disease [168]. A study that randomized
patients to V/Q scan versus CTA found PE to be diagnosed with
greater frequency in the CT arm, but that there was no
difference in patient outcomes or recurrent thromboembolism
during a 3 month followup period (i.e.: increased detection, but
no improvement in outcome) [168,169]. This suggests that CTA may
be identifying a milder, less fatal form in the spectrum of PE
(specifically subsegmental PE) [169,170]. With increased
identification of PE, there has come an increase in
complications from anticoagulation [170]. Some authors suggest
that patients with good cardiopulmonary reserve and self-limited
venous thromboembolism risk factors may not require
anticoagulation for subsegmental PE (assuming there is no
evidence of DVT) [168]. Interestingly, studies have shown that
lower extremity DVT is uncommon in patients with small
subsegmental PE, but can be found in up to 58% of patients with
central PE [170].
The clinical diagnosis of pulmonary embolism is unreliable-
symptoms of PE include tachypnea/dyspnea (most common [190]), pleuritic chest pain (next most common
[190]), tachycardia, hypoxia, hemoptysis,
syncope, and atrial fibrillation.
The pulmonary artery [pressure does not rise until greater than
30% of the pulmonary circulation is obstructed and it is arond
this threshold that PE becomes hemodyanmically important [190].
As the pulmonary artery pressure rises, the RV undergoes a
compensatory dilatation [190]. Massive PE may be associated with
cor pulmonale
and the ECG may show right axis deviation, P-pulmonale, RBBB, or other evidence of
right heart strain/dysfunction due to RV pressure overload
[137]. As the RV intramural pressure increases, this results in
decreased coronary blood flow [190]. The dilated and
dysfunctional RV also displaces the interventricular
septum toward the LV [137]. The extent of vascular occlusion
contributes to decreased venous return to the left atrium and
ventricle [137]. These changes eventually lead to acute
decreased cardiac output, hypotension, and shock [137]. RV
dysfunction seen at echocardiography is associated with a
2.4-fold increase in short term mortality [190]. Elevated
troponin and brain natriuretic peptide are associated with a 4-8
fold, and 6 fold increase in short term mortality, respectively
[190]. In patients with massive PE, approximately 65% will die
within one hour, and 93% within the first 2.5 hours [137]. Other
factors associated with an increased 30 day mortality risk
include age over 80 years, the presence of heart failure or
pulmonary disease, HR > 110 bpm, systolic BP < 100 mmHg,
and oxygen saturation on room air of less than 90% [190].
Thrombus in the right or left main pulmonary arteries correlates
with RV dysfunction and predicts a higher rate of 30-day
mortality or clinical deterioration [190].
A normal arterial blood gas does not exclude the presence of a
PE- in fact between 10-15% of patients with pulmonary embolism
will have a pO2 over 85 mmHg. Similarly, a low arterial pO2 is
non-specific. However, in patients with PE, an oxygen saturation
of less than 90% is associated with a greater 30-day mortality
risk [190]. In the general population, unsuspected
incidental pulmonary embolism can be found in 1.0% to 3.4% of
routine helical CT scans (outpatient 0.9%; inpatients 4%) [24,84,100,104,188]. The prevalence is
higher in patients with underlying malignancy (overall 2.6% to
4% of oncologic patients- 3.8% outpatients and 6% inpatients)
and inpatients (4%) [24,104,117,188]. Of all incidental PEs,
approximately 11-27% are confined to the subsegmental vessels
[188]. The clinical significance of these pulmonary emboli in
asymptomatic patients is uncertain, but in one study they did
not seem to be associated with an adverse outcome if not
identified [100]. The other point to remember is that patients
who survive the initial embolic event and are referred for
diagnostic evaluation form a different patient chort and it has been suggested that
mortality in this population may be as low as 5% [134]. It is
also important to remember that the risk of major bleeding while
on anticoagulation is for any VTE is 7.2 per 100 patient years
[188].
Although PE occurs most commonly from deep venous thrombosis in
the lower extremity, about 10% arise from clot in the upper
extremity primarily associated with an indwelling catheter. In
the PIOPED study, 92% of the patients with pulmonary embolism
had at least one of the following risk factors: Immobilization,
Recent Surgery, Underlying Malignancy, Genetic conditions
(Factor V leiden mutation, prothrombin 20210A mutation, and
other thrombophilias), History of Deep Venous Thrombosis or
Pulmonary Embolism, Estrogen use or Hyperestrogenic state, or
Pre-existing cardiac disease [2, 190]. Patients with
malignancies have a fourfold increased risk for developing
venous thromboembolism (VTE) and a
sixfold risk when receiving
chemotherapy [136]. Thromboembolism
is a known indicator of occult malignancy which can be found in
about 10% of idiopathic VTE patients [136]. HIV infected
patients are also at increased risk for PE due to associated
coagulation abnormalities [96,163]. The incidence of
thromboembolic events in HIV patients is reported to be
0.26-7.6%, and the incidence is higher in patients receiving
HAART or with underlying malignancy [163]. Patients with
nephrotic syndrome are at increased risk for PE which can be
seen in up to 30% of patients and the majority (84%) are
asympomatic [182]. Membranous nephropathy is the most common
form of nephrotic syndrome associated with PE [182].
Only about 10% of cases of pulmonary embolism result in pulmonary infarction, due to the presence of the bronchial circulation [3].
Certain clinical variables can be used to better stratify
patients that may require further evaluation for pulmonary
embolism [127,157].
|
Variables used to
determine patient pretest probability for
pulmonary embolism (Wells Criteria) |
|
Clinical signs and
symptoms of deep vein thrombosis (minimum of leg
swelling nad pain with
palpation of deep veins) : 3 points Pulmonary embolism as or more likely than an alternative diagnosis: 3 points Heart rate greater than 100/min: 1.5 points Immobilization (bed rest except for bathroom access for at least 3 consecutive days) or surgery in the past 4 weeks: 1.5 points Previous objectively diagnosed DVT or PE: 5 points Hemoptysis: 1.0 points Malignancy (on treatment or in the last 6 months or palliative): 1 point |
|
*Low risk <2,
moderate 2-6, high >6; PE unlikely if score <
4.5; PE likely if score ≥ 4.5 |
Unfortunately, one study demonstrated an artificial elevation
in the subjective component of Wells score by ED physicians when
the score became a required field for exam approval by
computerize physician order entry [185]. Other authors have used
other risk factors to determine the pre-test likelihood for PE:
immobilization, malignancy, hypercoagulable
state, excess estrogen state (pregnant, peripartum,
oral contraceptive use, or hormone replacement therapy), and a
history of prior thromboembolism
[152]. These authors found that PE was very unlikely (0.95%
chance) in the setting of no risk factors [152]. However, other
authors indicate that up to 30% of patients presenting with PE
have no predisposing facts [157]. The revised Geneva score can
also be used for patient risk stratification [185].
Revised Geneva Score [186]
- Age 65 years or over = 1
- History of previous venous thromboembolism: DVT or PE = 3
- Recent surgery or fracture within 1 month = 2
- Active malignant condition = 2
- Unilateral lower limb pain = 3
- Pain on deep palpation of the lower limb and unilateral oedema = 4
- Haemoptysis = 2
- Heart rate
- 75 - 94 bpm = 3
- 95 bpm or more = 5
Probability of pulmonary embolism:
- low probability 0-3 points
- intermediate probability 4-10 points
- high probability >10 points
The D-dimer blood test is another screening tool for pulmonary embolism that can be used in conjunction with clinical risk stratification of patients with suspected PE [145]. D-dimer is a cross-linked fibrin degradation product and a plasma marker of fibrin lysis [145]. Elevated levels of D-dimer usually occur with acute thromboembolic events and a negative result has a high negative predictive value [133,145,157]. The d-dimer test should always be used in combination with a clinical pretest probability assessment [138]. A D-dimer assay should be ordered only for patients with a low to intermediate clinical probability for PE [134,138]. When the D-dimer is negative in a patient with a low clinical probability of PE, the presence of acute PE can be safely ruled out without diagnostic imaging [145]. High risk patients would require additional evaluation regardless of the d-dimer result [134,138]- in one study, up to 9.3% of patients with a normal d-dimer assay, but a high clinical probability assessment, were found to have venothromboembolic disease [138]. Factors that have been suggested to contribute to false negative d-dimer results are recent administration of low molecular weight heparin (or anticoagulation), symptoms for more than several days, and small emboli [138]. However, exceptions to these suggested etiologies are not uncommon [138].
Assays for D-dimer can be divided into 3 groups:
1- Rapid quantitative ELISA assay which has a high sensitivity (over 95%) and negative predictive value, 95-100%, but low specificity [97,108,157]
2- Latex agglutination tests which tend to have somewhat lower sensitivity, but are more specific [97]
3- SimpliRED assay, a whole blood test that can be performed at the bedside with a pooled sensitivity of 87.5% and specificity of about 77% [97].
In general, a serum level less than 500 ng/L
excludes pulmonary embolism with a 90-95%
accuracy [1]. For patients with a normal rapid ELISA assay, the
likelihood for PE is 0.5% to 2% patients when the clinical risk
assessment for PE is low (4-15% likelihood by clinical
assessment) [108,122,134]. Therefore, patients with a low or
moderate clinical suspicion and a negative D-dimer exam will generally not require
further evaluation to exclude pulmonary embolism [76,91,94,119,122,130]. Unfortunately, a
positive test is non-specific (specificity is only 25-67%) and
occurs in 40-69% of patients [1,41,62].
Additionally, the test is unreliable in the presence of
malignancy, sepsis, recent surgery, pregnancy, or trauma which
can also produce elevated D-dimer
levels [1,97]. Normal D-dimer levels are also uncommon in
patients with a history of PE or DVT, elderly patients (over the
age of 80 years), and hospitalized in patients [157]. In one
study, the prevalence of PE increased with higher d-dimer levels and that study found that
the combination of a Wells score of less than 4 and a d-dimer level of less than 1000 ng/mL had a
negative predictive value of 100% for PE [159]. Adjustment of
the d-dimer level to account for patient age has also been
proposed as a mechanism to the diagnostic yield of CT PE imaging
[180]. For patients over the age of 50, the value for an
abnormal d-dimer level can be adjusted to age x 10 without
significant effect on the failure to detect PE (the failure
rate- defined as VTE within 3 months, was only 0.3% compard to a
failure rate of 0.5% for patients that had underwent CTPE
imaging) [180].
Massive pulmonary embolism has traditionally been defined as a
50% or greater obstruction of the pulmonary vasculature or
occlusion of two or more lobar arteries [137]. Another finding
that suggests massive PE is one that causes a sustained
hypotension (<90 mmHg) for more than 15 minutes or requires
vasopressor support [190]. The three month mortality rate from
PE has been reported to be up to 58% for hemodynamically
unstable patients at presentation, compared to 15% for patients
that were hemodyanmically stable [191]. A submassive PE is one
that causes right heart strain, dilatation, dysfunction, or
ischemia; elevation of brain natriuretic peptide or troponin;
new or complete RBBB, anteroseptal ST changes or anteroseptal
T-wave inversion [190]. Low risk PE is defined as having no
associated hypotension or right heart dysfunction or dilatation
[190]. Patients that present with hemodynamic instability
have a higher mortality rate of 50-58% [95]. Acute embolic
obstruction of more than 30% of the pulmonary circulation
increases pulmonary vascular resistance and leads to acute
pulmonary arterial hypertension [95]. This is worsened by the
release of vasoactive agents from
plasma and platelets, and reflex PA vasoconstriction leading to
systemic arterial hypoxemia [95]. Clinical signs of right heart
dysfunction include tachycardia, low arterial blood pressure,
distended neck veins, accentuated pulmonic
second heart sound (P2), and tricuspid regurge murmur [95].
In 2014, the European Society of Cardiology proposed a system
that classified PE as high risk if associated with hypotension,
intermediate-high risk if associated with both clinical AND
cardiac biomarker evidence of RV dysfunction without
hypotension, and intermediate-low risk if associatd with either
imaging or cardiac biomarker evidence of RV dysfunction without
hypotension, and low risk if associated with none of those
findings [191]. Cardiac biomarkers of RV dysfunction include
elevated troponin or B-natriuretic peptide (BNP) and imaging
evidence includes RV/LV diameter ratio > 0.9 or RV
dysfunction on cardiac echo (RV hypokinesia, RV dilatation, or
increased tricuspid regurgitation jet velocity [191].
Thrombolytic agents (such as urokinase
or rtPA [recombinant tissue-type plasminogen activator]) are not
routinely used for the treatment of acute PE. Clinical trials
have demonstrated that thrombolytic therapy produces more rapid
clot lysis and more rapid
improvement in hemodynamics than
anticoagulation therapy alone [36]. However, in hemodynamically stable patients no
difference in patient mortality or the incidence of recurrent PE
has been demonstrated [36]. Thrombolytic therapy is also
associated with a higher risk for major hemorrhagic
complications (12% incidence) and intracranial hemorrhage (1.2%
incidence) [36,191].
Thrombolytic treatment is generally reserved for patients with
massive/high risk pulmonary embolism producing circulatory shock
(hypotension) and has been shown to signifcantly reduce
mortality [30,190,191]. Thrombolytic therapy is most effective
when administered soon after PE and its effectiveness decreases
with increasing symptom duration [36]. Surgical embolectomy can
be considered for high-risk patients if clinical instability
persists after the administration of systemic thrombolysis
[191]. Percutaneous catheter directed thrombus removal with or
without catheter directed thrombolysis can also be considered
for patients with high risk PE [191]. Percutaneous
catheter-directed thrombolytic treatment or surgical embolectomy
may be considered for intermediate-high risk patients if
hemodynamic decompensation appears imminent and the anticipated
bleeding risk for systemic thrombolysis is high [191].
Treatment for PE most commonly consists of anticoagulation with heparin or coumadin. Anticoagulation prevents clot propagation and allows endogenous fibrinolytic activity to dissolve existing thrombi [36]. Without anticoagulation therapy, PE has an estimated mortality rate of 30-36%, but with anticoagulation it has a mortality rate of 2.5% [30]. The risk of major hemorrhage with therapeutic heparin is 3-8% [30]. Other authors suggest that the risk of a major hemorrhagic complication from heparin therapy is actually closer to 1.8% [36]. The rate of major bleeding caused by warfarin has been reported as less than 3% and the mortality rate less than 0.5% at 3 months [87].
For patients that cannot be anticoagulated,
an inferior vena caval filter can
be placed in order to prevent life-threatening PE. Major
complications occur in about 1% of cases. Complications include
central migration of the filter, filter fracture, inferior vena
caval perforation, and vena caval thrombosis [48].
Pulmonary embolism in children:
Infants and neonates are the age group at the greatest risk
[183]. Children with PE have an identifiable risk factor 96-98%
of the time [183]. Neonates typically have several simultaneous
risk factors including dehydration, septicemia, peripartum
asphyxia, and a central venous catheter with associated thrombus
is present in 80% of cases [183]. In older children and
adolescents, a central venous catheter is the single greatest
risk factor [183]. Other risk factors include malignancy, lupus,
renal disease, cogenital thrombophilia, surgery, major trauma,
and immobilization [183]. Because of excellent cardiopulmonary
reserve, even large PE may cause only subtle clincal signs and
symptoms [183]. Emboli that obstruct less than 50% of the
pulmonary circulation are often clinical silent [183].
Other emboli:
Pulmonary cement embolism can be seen in 2.1-5% of patients
after percutaneous vertebroplasty or kyphoplasty
(up to 23% after percutaneous vertebroplasty in patients with
osteoporotic vertebral compression fractures) [79,189]. The risk
of embolozation is increased if perivertebral venous leak occurs [79].
The emboli appear as dense tubular and branching opacities on
CXR [79].
Air embolism can lead to mechanical obstruction of the
pulmonary vasculature, however, the lethal volume of air is
about 300-500 mL injected at a rate of 100 mL/s [189]. Patients
with air embolism should be placed in theTrendelenburg position
and left lateral decubitus position [189].
X-ray: View cases of pulmonary embolism
Ventilation-perfusion scintigraphy:
See also discussion in nuclear medicine V/Q section
V/Q scanning had been the mainstay for screening symptomatic patients for the presence of pulmonary embolism, but it's used has decreased significantly since the introduction of MDCT [119]. The problem with V/Q scanning is that it does not directly visualize thromboembolism, but rather its effects on perfusion and ventilation [47]. This problem causes the need for probability criteria, which in turn causes confusing results and high interobserver disagreement [47]. A negative V/Q scan essentially excludes PE, and a high probability study is associated with the presence of a PE in about 85% of cases at angiography. Unfortunately, in the PIOPED study only 14% of patients studied had a normal exam and 13% had a high probability exam [90]. Confusion arises with low or intermediate probability examinations (which accounted for 73% of exams in the PIOPED study [90]), and there is a 25%-35% disagreement among expert readers in the interpretation of scans in these categories. Performance of V/Q imaging was better in the PIOPED II trial. In that study, 56% of patients had a very low probability V/Q scan [119] and 73.5% of patients had diagnostically definitive imaging when using PE present (high prob) and PE absent (very low prob or normal) exam interpretations [132]. This may be because PIOPED II included a large number of outpatients for imaging (critically ill in-patients are more likely to have scans that are difficult to interpret) [132].
Nuclear medicine scanning for PE is probably most useful in previously healthy patients with a normal chest radiograph [38,46]. Up to 91% of patients with normal CXR findings have been shown to have a diagnostic V/Q scan [122]. As the complexity of the patients underlying cardiopulmonary disease increases, so does the likelihood that the scan will not be informative (intermediate probability) [64]. Using PIOPED criteria, intermediate probability V/Q scans occurred in 60% of patients with COPD, but in only 13% of patients with normal CXR's [64]. However, a generalized abnormality on CXR, such as diffuse pulmonary edema or reticulonodular disease, may not cause the perfusion lung scan to be abnormal- in fact, up to 73% of patients with such findings can have normal or near normal perfusion images [64]. V/Q imaging is also indicated in patients with iodinated contrast material contraindications such as renal failure or dye allergy. It has been suggested that women of reproductive age should undergo a V/Q scan rather than a CT study if their clinical pretest likelihood for PE is low (as graded by an experienced clinician) [119] or when the CXR is normal and there is no clinical suspicion of an alternative diagnosis [154]. Both exams are likely to exclude PE with the same certainty, but with less radiation risk from the V/Q study [119,139] (CTPA and V/Q scanning have equivalent clinical negative predictive values [154]), but the effective radiation exposure from a CTPA exam is 5 to 7 times that of a V/Q scan and there is about a 20-40 fold higher dose to the female breast [161,170] ). Breast irradiation from a V/Q scan is approximately 0.28-0.9 mGy - which is less than 5% of the radiation dose to the breast resulting from CTA [139]. Even a non-diagnostic V/Q scan in combination with a negative lower extremity venous US essentially excludes PE if the clinical suspicion is not high [127].
One additional useful purpose of the V/Q is that is can be used to follow-up patients with positive CT PE exams [130]. By obtaining a baseline V/Q scan after a positive CT PE study, followup patient evaluation can be performed using V/Q imaging in order to decrease patient radiation exposure [130].
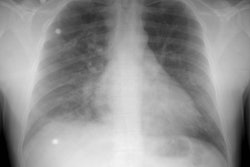
Plain film:
The CXR is abnormal in the majority of cases of PE. The PIOPED study showed that among patients with angiographically proven pulmonary embolism, only 12% had chest X-rays interpreted as normal [4] (24% of patients with PE in another study had normal CXR's [50]). Atelectasis and other focal pulmonary parenchymal abnormalities are the most common CXR findings in pulmonary embolism, occurring in up to 68% of patients with PE (cardiomegaly was the most common finding in another study [50]). Pleural effusions are also common (23% of patients in one study [50]), usually small, unilateral, and occupy less than 30% of the hemithorax [5]. Other palin film findings indicative of PE include regional oligemia beyond the occluded vessel (Westermark sign), a pleural-based wedge shaped area of increased opacity (Hampton's hump), and prominence of the central pulmonary artery (Fleischner sign) [22]. The Palla sign is related to the swollen interlobar artery that has a bulging contour mimicking a sausage appearance [190].
Angiography:
Patients are studied one lung at a time with an initial biplane (AP and lateral) run, followed by a contralateral oblique study. Contrast is 60% non-ionic injected at 25 cc per second for a total of 40 cc for each run. Angiography is actually a relatively safe procedure with a major complication rate of under 2%. Yet, at many institutions angiography is used in less than 15% of unresolved cases for pulmonary embolism [6]. Patients that should be considered to be at greater risk for complications include: intensive care unit patients, patients with tenuous right ventricular function (ie: pulmonary hypertension with pressure above 20 mmHg), and those with left bundle branch block (the procedure may produce a right bundle branch block and result in complete heart block). In patients with pulmonary arterial hypertension the necessity of the procedure should be reviewed or subselective injections only performed.
Classically, a embolus produces a filling defect within the affected pulmonary artery. Non-occlusive emboli have a "tram-track" appearance. Although considered the gold standard, angiography may not always detect the presence of emboli. Some indirect angiographic evidence for the presence of emboli such as vascular pruning and delayed capillary blush are non-specific. Additionally, agreement among angiographers regarding the presence of subsegmental emboli is poor (66% of cases in the PIOPED study [31]) and can be as low as 15%. V/Q scans can provide a road map to angiography, but if the abnormally perfused segment on the V/Q scan appears normal at angiography, complete evaluation the remainder of the lungs for the presence of pulmonary emboli is warranted. One important point to remember is that a negative angiogram has been shown to be an excellent indicator of a good prognosis [7].
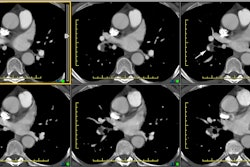
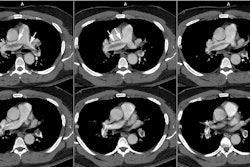
Helical CT:
General:
Up to 30% of patients evaluated for PE will have intermediate
probability V/Q scans and negative lower extremity duplex exams
for deep venous thrombosis [19]. Ultimately, between 20-30% of
these patients will be shown to have PE at angiography.
Unfortunately, despite the relative safety of pulmonary
angiography, many physicians are reluctant to proceed to this
step. With advances in helical CT the role of computed
tomography in the diagnosis of PE may has dramatically changed.
The Fleischner Society has
recommended that helical CT should be the initial imaging
modality to evaluate patients suspected of having PE if there
are no contraindications to the exam (such as iodine allergy or
renal impairment) [122]- particularly in patients with abnormal
CXR's in whom there is a greater likelihood of inconclusive V/Q
scan results [46]. Helical CT is able to identify main, lobar,
and segmental emboli with reported sensitivities greater than
90%. Emboli as small as 2 mm in the 7th
order vascular divisions have been detected. Although
the detection of subsegmental
emboli is lower, the clinical significance of these small emboli
has not yet been established. Additionally, on angiography there
is poor interobserver agreement for
the presence of subsegmental emboli
[31] and the true incidence of isolated subsegmental
emboli is difficult to determine. Helical CT has been shown to
have a significantly better sensitivity, specificity, positive,
and negative predictive values compared to V/Q scanning [44,73]. Helical CT has greater
discriminatory power and permits a more confident diagnosis to
be made in a greater number of cases when compared with V/Q
scanning [44,88]. Another benefit of
CT is the ability to suggest an alternative diagnosis in 11-57%
of patients to explain their clinical symptoms [19,23,32,37,66,73]. Utilization of CT for
the evaluation has increased dramatically since the introduction
of multidetector CT scanners [78].
The prevalence of PE in the PIOPED study was 33%- the prevalence
of PE in patients sent for CT angiography can be as low as 5-12%
[78,123,168]. At least one study found that up to 32% of all
CTPE examinations are potentially inappropriate [187].
Computerized physician order entry systems that incorporate
clinical decision support (CDS) can be used to improve
appropriateness and the yield of CTPE imaging [187]. However,
the ability of physicians to simply override CDS recommendations
has been shown to result in lower exam yield and fails to
optimize provider behavior [187]. Stronger interventions that
implement physician performance feedback reports or requiring
real-time peer-to-peer consultation before ignoring CDS alerts
have been shown to result in increased adherence to evidence
presented in CDS [187].
Multi-detector Helical CT exam:
Hyperventilation before the start of the exam (consisting of a few deep breaths) is recommended as it will facilitate prolonged breath holding. The breath-hold required for 16-MDCT is about 10 seconds and for 64-MDCT less than 3 seconds [115]. Patients unable to maintain a breath hold can be scanned while gently breathing to reduce respiratory motion artifacts. Patients on a respirator can be imaged during a forced period of apnea or while breathing at a minimal tidal volume and respiratory rate [18].
The scan is performed during an infusion of 80-120 ml of 30%
contrast material (3-5 ml/sec) with a 15-20 sec scan delay (some
centers use 60% iodinated contrast and a lower injection rate).
Ideally, the empiric delay should be adjusted for the type of
scanner (16 versus 64 slice) and the
contrast injection rate [115]. Alternatively, a small test
injection (15-20 cc) can be used to determine optimal timing of
the scan- this is particularly beneficial in patients with known
or suspected cardiac dysfunction [90]. The time to peak
enhancement plus 5 seconds is used as the time delay for the
diagnostic scan. The extra 5 seconds allows for opacification of the distal small
arteries and provides a margin for error. For patients with
right ventricular failure or pulmonary hypertension a longer
scan delay is required (15-18 seconds) [37]. Bolus tracking with
an ROI over the main pulmonary artery is another method to
determine the proper scan delay- the scan is triggered when the
measured contrast enhancement exceeds a pre-set threshold [115].
However, some authors feel that bolus tracking results in the
worst pulmonary artery enhancement and favor an empiric delay as
it allows better patient breath hold timing [115]. The theorectic minimum vascular attenuation
required to see all acute and chronic pulmonary thromboemboli are 93 and 211 HU
respectively [115]. Some authors suggest that adequate image
quality can be obtained using only 30 mL of IV contrast and
64-slice MDCT imaging and this can be of tremendous value for
the evaluation of patients with renal dysfunction [173].
A patients body weight and the amount of contrast medium injected are closely related to the degree of enhancement [114]. To achieve a consistent degree of contrast enhancement, the amount of contrast can be adjusted for the patient's body weight and the volume of contrast can be decreased for greater detector scanners (64 versus 16-slice) due to shortening of the scan duration [114]. For contrast containing 350 mg of iodine per milliliter, the dose of contrast is 1.2 mL per kg of body weight (0.4 gm of iodine per kg of body weight) [114]. The volume of contrast injected should correspond to: (injection rate) x (scan delay + the scan duration) [114]. In this study, no patient received more than 125 mL of contrast (the largest volume that could be held by the injector) [114]. To simplify this method, 110 mL of 370 mgI/mL can be used for patients less than 250 lbs, and 130 mL can be used for those over 250 lbs [115]. The volume of contrast should be reduced to 70 mL in pregnant patients as the legs and pelvis will not be imaged and the quantity of iodine to the fetus is also decreased [115].
Scanning caudo to cranial and using a lower injection rate reduces streak artifacts from concentrated contrast material in the superior vena cava which may obscure the adjacent right main pulmonary artery. Also, caudo-cranial imaging will minimize motion artifacts due to respiration that tend to be greatest in the lung bases and less significant in the upper lungs [115].
Multi-detector helical CT is superior to the single detector examination for the evaluation of sub-segmental pulmonary arteries [59,63,65]. With a 16 to 64 detector CT scanner, the entire chest can be imaged in a short (10 second or less) breath hold using 1 mm or sub-millimeter resolution [74]. Use of thinner sections (1 to 1.25 mm) results in substantially higher detection rates for subsegmental emboli (especially for obliquely oriented vessels), fewer inconclusive interpretations, and better agreement among readers [65,70,73,86]. Up to 74% of fifth-order arteries can be identified when using a section thickness of 1.25 mm [59]. The main reason for inadequate detection of these small vessels is partial volume effects, cardiac pulsations, and respiratory motion [59]. Up to 40% more subsegmental emboli can be detected on 1 mm sections compared to 3 mm sections and indeterminate readings can decrease by up to 70% [65,87]. However, interobserver agreement does decrease with smaller vessels [90]. When using a multidetector CT, retrospective ECG gating can be applied to reduce transmitted pulsation artifacts [74]. The detection of smaller emboli comes at the price of increased patient radiation exposure [65]. A multidetector scan using 1 mm collimation results in about a 25% increase in effective radiation dose compared with 5-mm collimation using a single detector CT [65]. A fixed scan delay prior to imaging may result in suboptimal vascular opacification. To determine optimal timing for image acquisition, a test bolus or bolus tracking can be performed. Bolus tracking does result in an additional radiation dose to the patient [111]. Assuming 10 monitor images (using 140 kV, 43 mA, and 0.75 sec rotation time) the patient would receive an additional effective dose of 1.4 mSv [111], but this would be isolated to the section being imaged.
Modulation of the exams acquisition parameters based upon patient body habitus can aid in reducing radiation dose [74].
Gadolinium enhanced MDCT exam:
In patients with a history of severe contrast allergy or renal insufficiency diagnostic quality MDCT examinations for PE can be performed using gadolinium as the contrast agent [99]. A dose of 0.4 mmol/kg should provide overall good image quality in most patients [99]. High injection rates are required (6 ml/sec) and scanning should be initiated using automatic bolus triggering centered on the main pulmonary artery trunk with a threshold of 50-70 HU [99]. Improved pulmonary artery enhancement can be obtained by using 80-100 kV for scanning, rather than 120 kV [99]. Transient impaired renal function has been described in patients with underlying renal disease following the exam [99].
Radiation exposure:
Overutilization of CT angiography for the evaluation of
suspected PE has lead to concerns regarding radiation exposure.
This is also a concern as many patients will undergo more than
one CT examination (30% of patients that had CT for PE
evaluation underwent at least 3 examinations, 7% at least five,
and 4% at least nine) [170]. Despite this increased utilization
of CT, the likelihood for a positive exam is decreasing [170].
In one study, 92% of female patients had CTPE exams negative for
PE [152] and others report only about 5% of exams are positive
for PE [168]. Approximately 20% of ED patients who undergo
pulmonary CTA are women of childbearing age (and glandular
breast tissue is especially susceptible to ionizing radiation)
[172]. An average sized woman's breasts receive between 2.0-4.0
rad (20-40 mGy)
of radiation from a thoracic CT exam, but the dose can be as
high as 6-8 rad (60-80 mSv) in a woman with large breasts
[93,102,120,157,172]. This is equivalent to approximately
100-400 chest radiographs [134]. A standard two view mammogram
is associated with an average breast dose of 3 mSv dose (0.300 rad
or 3 mGy) [93,130]. Therefore, the
dose from the CT exam is equivalent to 10-25 two-view mammograms
[93] and considerably higher (70-100 times) than the absorbed
dose to the breast from perfusion scintigraphy-
approximately 0.028 rad (0.28-0.9 mSv or 0.28 mGy)
[108,130]. Even when using 256 slice low-dose CTPA, the
effective maternal dose is 30% higher than V/Q imaging (the
fetal dose is 3.4-6 times lower, but the fetal dose from V/Q is
still very low) [181]. The effective dose from CTPA is also
strongly dependent on patient body size, as the effective dose
has been shown to triple as BMI increases from 19.7 to 30.1 kg/m2
[181]. Fetal dose also increases with icreasing BMI and
increases 25-80% as pregnancy progresses- likely related to
fetal growth and the embryo approaching the exposed body region
[181].
The potential latent carcinogenic effects of this radiation is not definitively known. The estimated additional relative risk of all cancers from the International Commission of Radiologic Protection is 0.005% per millisievert of dose [168]. Other data suggest that 0.1 rad of radiation exposure may lead to 5 additional cancers in 100,000 exposed patients [93]. Assuming a linear relationship between increasing radiation dose exposure and the stochastic effects of ionizing radiation on biologic tissue, one can extrapolate a possible additional 100 cancers per 100,000 exposed individuals from CT PE evaluation [93]. The Fleischner Society states that the radiation exposure from one spiral CT would result in approximately 150 excess cancer deaths per million people exposed [122]. Other authors have estimated that the delivery of 1 rad of radiation to a woman's breasts before age 35 years may fractionally increase her risk of breast cancer by 13.6% over the expected spontaneous rate for the general population [37]. Other authors suggest that the additional lifetime risk of radiation induced breast cancer from a single CT PE exam could be in the range of 1 in 500 to 1 in 5000 [119]. One point to remember is that excess risk for malignancy is related to the age and sex of the patient- those at the greatest risk are girls and young women aged 15-25 years [126,168,169]. Thus- the increase in lifetime attributable risk of cancer is 1 in 143 for a 20 year old woman and 1 in 284 for a 40 year old woman [130].The risk for radiation induced cancer would be increased in an additive manner if multiple follow-up exams were performed [126].
One important point to remember about radiation risks is that data has been extrapolated from Japanese atomic bomb survivors [135]. Those individuals received radiation at very high dose rates, the total doses included a significant neutron component, and the subjects were also exposed to fallout that resulted in internal radiation doses [135]. Thus- it remains an assumption that radiation from diagnostic imaging can be carcinogenic- however, it remains prudent to limit the amount of radiation a patient receives for any particular study [135]. Also- the small potential risk of cancer induction must be considered in the context of the potential incremental survival benefit from the identification of PE on the CT examination [144].
Also- studies have shown that at least one-third of patients who undergo one CT angiographic exam for suspected PE will undergo a second CTPE exam within 5 years with a positive yield rate as low as 3.5% [151]. Twenty-two percent of patients who undergo repeat CTPE have been found to be women under the age of 40 years [151].
Dose reduction can be performed for the CT PE exam, but care
must be taken not to decreased image quality which can result in
decreased detection of pulmonary emboli and an adverse effect on
diagnostic confidence [128]. Some authors suggest only slight
dose modification for patients over the age of 60 years, but
more effective dose adjustments for very young patients [175].
There are several ways to decrease the radiation exposure from
the exam:
One of the simplist methods to reduce radiation exposure is by
adjusting the scan length [175]. It has been suggested that
adjusting the scan length from just above the aortic arch to
just below the heart will maintain 98% diagnostic accuracy with
a dose reduction of 37% [175].
Dose modulation: The body is not a perfectly round object and
there are varying degrees of attenuation through the chest
(higher at the shoulders and less in the lower chest [175].
Optimal image quality is achieved when all projections have
comparable numbers of photos detected [175]. Dose modulation is
available on many CT units and automatically adjusts the dose to
minimize exposure [123]. The mean radiation dose can be
decreased 15-20% at the level of the upper chest and by about 5%
at the level of the middle and lower chest with the use of dose
modulation [123]. There are two approaches to dose modulation-
in the first, relative attenuation is determined by two prescan
planning digital radiographs of the area to be imaged [175]. The
z-axis modulation is planned from the frontal view and the x-
and y-axes from a combination of the frontal and lateral views
[175]. In the second approach, z-axis modulation is determined
from a frontal prescan and the x- and y-axes modulation is
determined by real time monitoring of the attenuation by the
rpevious x-ray tube rotation [175]. Organ-based tube current
modulation is another method to reduce direct exposure of
superficial radiosensitive organs [176]. In this method, the
tube current is reduced when x-rays pass through the patient
from anterior to posterior, but increased for the other
projections (posterior to anterior) to maintain image quality
[176].
Low kVp: A low kVp technique (100 kVp) can also decrease radiation exposure by more than 44-50% [110,143,172]. However, decreasing kVp from 120 to 100 results in an increase in background noise of 19% [123]. Despite this reported increase in image noise, lowering kVp does not produce significant loss of objective or subjective image quality [123,172]. This is because, although lower kVp settings can be associated with increased image noise, the technique actually results in improved vessel opacification and an increase in mean image signal [110,143,172]. This is because the effective energy of the x-ray beam decreases and comes nearer to the maximum absorption (k-edge) of iodine [110]. A low kVp technique may be most applicable to patients that weigh less than 100 kg (220 lbs) [172]. Another method is to adjust the kVp based on the patient's body mass index (particularly for younger patients) as follows: BMI < 20, 80 kVp; BMI 20-25, 100 kVp; and BMI > 25, 120 kVp [175].
Bismuth breast shields: During CT PE imaging, the mean
glandular dose to breast tissue may range from 20-60 mGy, and
the inferior aspect of the breast may receive approximately
10-20 mGy during abdominal CT [174]. Breast dose can also be
decreased 30-57% by the use of thin-layered bismuth radioprotective garmets
[93,158,174]. The use of shielding can produce beam hardening
streak artifacts and increased image noise, although these
artifacts are primarily in th region of the breast (1 to 3 cm
frm the shield), rather than in the diagnostic portions of the
images [165,174]. It is important to place the shield flat
without bunching or wrinkling [176]. A 1cm thick foam pad placed
on top of the patient can help to lift the shield away from the
chest wall and to keep the shield smooth in order to decrease
artifacts [176]. A similar shield placed over the thyroid gland
can decrease the thyroid dose by 60% [157]. The reusable breast
shields cost approximately $165.00 [93]. Note that the scout
view should be acquired prior to placement of the shield so as
not to affect proper dose modulation (otherwise the program
might offset the protective effect by increasing current to
maintain photon flux through the shield) [158,174]. Breast
shields should also not be used with CT systems that use
real-time tube current modulation [175]. It should be noted that
bismuth breast shields are associated with some wasted radiation
- whereas anterior exposure is substantially reduced, posterior
exposure is only minimally lower and the shield reduces the
transmission of useful photons in both the AP and PA directions
[174]. Whenever possible, z-axis tube current modulation should
be used in conjunction with breast shields [174].
Overall, the use of CTPA imaging has not resulted in improved patient outcomes from PE [147]. Although there is a higher rate of PE diagnosis when patients are imaged with CTPA compared to V/Q scanning, in one study there was no difference in mortality or complications due to thromboembolic events during a 3 month followup period (this suggest over diagnosis) [147]. Because of this and the higher radiation exposure from CTPA, it has been suggested that women of reproductive age should undergo a V/Q scan rather than a CT study if their clinical pretest likelihood for PE is low (as graded by an experienced clinician) [119]. Both exams are likely to exclude PE with the same certainty, but with less radiation risk from the V/Q study [119]. The total effective radiation dose from CTPA is approximately 5 times greater than that from V/Q imaging [147]. Breast irradiation from a V/Q scan is approximately 0.28-0.9 mGy - which is less than 5% of the radiation dose to the breast resulting from CTA (the breast dose is 20-40 times greater from CTPA) [139,147]. The Fleischner Society recommends V/Q imaging with lower extremity US in women of reproductive age with positive D-dimer assays being evaluated for PE, but also indicates that CT angiography and lower extremity US are acceptable alternatives if the clinical situation indicates it [122]. They do recommend that if CT DVT is deemed necessary that the pelvis not be imaged in order to reduce gonadal radiation [122]. [126]. Another group of patients that could benefit from V/Q imaging and lower radiation exposure are patients with a normal CXR [147]. Patient outcome following V/Q imaging are similar to CTPA, with three series showing a less than 1% incidence of serious thromboembolic events over a 6 month or longer followup period after a low-probability V/Q exam [147].
In pregnant patients, the mean fetal dose with single-detector CT has been shown to be less than that for V/Q scanning [90]. The dose from V/Q scan ranges from 100-370 mGy, while the fetal dose from CT is 3.3-20.2 mGy (first trimester), 7.9-76.7 mGy (second trimester), and 51.3-130.8 mGy (third trimester) [90]. V/Q scanning, however, does not require contrast administration and with CT there is the potential for contrast reactions.
Sensitivity/Specificity:
Early studies showed that single detector helical CT had sensitivity for pulmonary embolism in a segmental artery or larger between 53% to 100% (helical CT has a higher sensitivity than V-Q scintigraphy [37]). Some of the lower sensitivity data reported is from early studies in which thicker collimation (5mm) was utilized. Multidetector helical CT is superior to single detector helical CT in the detection of pulmonary embolism with reported sensitivities of 83-100% and specificities of 78-97% for the detection of pulmonary embolism [82,112,121,122]. In the PIOPED II study, CT angiography had a sensitivity of 83% and a specificity of 96% for the diagnosis of acute PE (unfortunately, PIOPED II was primarily conducted with 4 to 16 slice CT scanners and may not represent the accuracy of 64-slice imaging [156]) [108,132]. The negative predictive value of CT PE is 81-100% and the positive predictive value is 60-100% for detecting emboli within the central pulmonary arteries [See multiple references below]. In PIOPED II the PPV was 96% with a concordantly high probability for thromboembolism on clinical assessment, but dropped to 58% if the clinical suspicion was low [156]. In another study, the overall PPV was only 74% [184]. The negative predictive value of a normal exam is variable as pulmonary embolism has been reported in up to 5.4%-15% of patients with normal helical CT scans [19,23,27]. This is higher than the 0.6% to 4.2% incidence of PE in patients with negative pulmonary arteriograms [19]. However, in recent studies, multidetector CT with 1.25 mm collimation has been shown to be significantly more sensitive than angiography in the detection of PE [121]. Multidetector CT has particularly improved PE detection at the subsegmental level [81] The likelihood ratio for acute PE is 19.6 with a positive MDCT exam, versus 0.2 with a negative study [119].
The utility of helical CT in the evaluation of critically ill patients has been questioned [60]. A lower sensitivity and specificity for detection of PE in a subgroup of critically ill surgical trauma patients has been suggested- possibly related to extensive underlying parenchymal abnormalities. Unfortunately, this study suffered from a very small sample size and no effort was made to suspend respiration during the examination which likely resulted in degradation of image quality [60].
One important point to remember is that the accuracy of CTPA imaging decreases when the findings are discordant with the clinical suspicion [147]. The PPV of a positive CTPA exam has been shown to be only 58% when the clinical probability is low, similar to the 56% found for V/Q imaging [147]. This is because for a given sensitivity and specificity, the NPV increases and the PPV decreases with a reduction in the prevalence of the condition in the study population [151]. Due to over-utilization, fewer than 10% of patients being evaluated for embolism from the ED setting are shown to have PE (i.e.: decreased prevalence) [151]. The PIOPED II concluded that the PPV and NPV of CTPE was high with a concordant clinical assessment, but that additional testing is necessary when the clinical probability is inconsistent with the imaging results [151].
When thin section CT PE images are coupled with a CT DVT exam
the study probably provides an excellent survey for clot.
None-the-less, further evaluation for pulmonary embolism using
angiography in selected cases following negative CT exams may be
reasonable in selected cases. Interobserver
agreement for helical CT is better than that for V/Q scanning
[44]. Interobserver agreement for
clot within lobar arteries is very good (92%), but agreement
decreases with more peripheral vessels and for the absence of
pulmonary embolism [29,49,67].
Computed aided detection has been applied to CT PE imaging and
has been shown to correctly identify up to 77.4% of acute PE's
that were previously missed in clinical practice [179]. On
avergae, CAD produces approximately 4 false-positive markes per
case (range 0-23) [179]. One potential concern with CAD is that
it may lead to an increased diagnosis and treatment of patients
with small subsegmental PE's (clots that might resolve without
treatment- clinically insignificant clots) [179].
Subsegmental emboli:
Although sensitivity is better for detection of central emboli, when subsegmental vessels are included, sensitivity decreases to 63-67% for conventional helical CT, while specificity ranges from 78-100% [9,11,23]. Improved evaluation of subsegmental arteries is obtained with the use of multidetector CT with thin slices (1-1.25 mm) and overlapping reconstructions. Theoretically, this loss of sensitivity should affect only those patients with isolated subsegmental emboli. Unfortunately, interobserver agreement among angiographers as to the presence of subsegmental emboli is poor [28]. The reported incidence of isolated sub-segmental emboli ranges from 1.0%, to as high as 36% (in a sub-selective group of patients referred for angiography) [9,10,13,28,30,45,53,66,73,82,86,91]. In the PIOPED study, 5.6% of patients enrolled in the study and 16% of patients with positive angiographic findings had isolated subsegmental pulmonary emboli (for patients with low probability scans, the incidence was 17%). However, in the PIOPED study, two readers disagreed on the diagnosis of subsegmental emboli 34% of the time [87].
To further complicate matters, the clinical significance of subsegmental emboli has not been fully described. Patients with untreated isolated subsegmental emboli may not be at increased risk for a poor clinical outcome [28,86], particularly if they have adequate pulmonary reserve [87]. The intrinsic fibrinolytic activity of the lung will resolve most small emboli spontaneously (however, DVT does not) [87]. In the PIOPED study, 20 patients had negative pulmonary angiography results at their local hospital and did not receive anticoagulation therapy [87]. Susequently, an expert panel determined that PE was indeed present in those cases [87]. Of the 20 non-treated patients one (5%) had a fatal embolus and one (5%) had a nonfatal embolus [38]. In anticoagulated patients in PIOPED the fatality rate was 2.5% and the recurrence rate was 3.5% [87].
However, small emboli may produce significant morbidity in patients with underlying cardiorespiratory disease [23,28]. Additionally, among stable patients small emboli may indicate a risk for recurrent more significant emboli. In these cases, lower extremity evaluation to exclude the presence of DVT would be mandatory [86].
Prognosis:
Although sensitivity and specificity data is important, one also needs to address the clinical outcome following a negative exam. Patients with negative helical CT exams have been shown to do well clinically [34,43,44,46,49,65,75]. In one retrospective study, no patient with a negative helical CT for PE subsequently developed an embolism and there were no patients deaths attributable to PE during a 6 month follow-up period [43]. In another study only 1 of 78 patients (1.2%) with a negative helical CT subsequently developed microemboli (detected at autopsy) [34]. Other studies have shown subsequent thromboembolic events in 0.8-5% of patients with negative CT PE exams [44,49,94,122,147]. Two prospective studies found a 1% incidence of subsequent pulmonary embolism in patients with negative helical CT PE exams [46,75]. In another study, PE occurred in only 2% of patients within one year following a negative CT PE exam [66]. In an analysis of MDCT, only 1% of patients with a negative study developed PE (nonfatal) within 6 months of the initial study [89]. A randomized controlled trial found a 0.4% incidence of thromboembolism during a 3 month follow-up after PE was initially considered excluded based upon the CT PE exam [127]. These data indicate a favorable outcome for patients with negative helical CT PE exams- particularly those with low to moderate clinical risk assessment. The rates of recurrent PE after a negative spiral CT exam are also similar to that reported following a negative pulmonary angiogram (0.8% to 3.5%) [66]. The 3 month rate of thromboembolic disease following negative V/Q imaging has been reported to be about 1.4% [127]. The likelihood for subsequent pulmonary embolism may even be less if the CT PE exam is coupled with a negative CT DVT exam. One author has concluded that in most patients with suspected acute PE and no symptoms of DVT, anticoagulation therapy can be safely withheld following a negative CT PE exam [90].
Note: In PIOPED II, for patients with a negative CTA and venography result, pulmonary embolism was found in 3% of patients with low clinical risk assessment, 8% with moderate probability assessment, and 18% of patients with high probability assessment [108].
Image interpretation:
Images should be reviewed on a workstation. The use of a modified window setting can aid in increasing vessel conspicuity (such as level 100 to -100 HU, width 700 to 1000 HU) [63,80]. The signs of PE include: 1- a central intravascular filling defect outlined by contrast material within the vessel lumen; 2- eccentric tracking of contrast material around a filling defect that forms acute angles with the vessel walls; 3- and complete vascular occlusion with failure of enhancement of the entire vessel lumen and possibly with enlargement of the involved vessel [80,106,115]. Filling defects that form a smooth, obtuse angle with the vessel wall usually represent chronic thrombi, but may represent the sequella of recent resolving emboli. The lung parenchyma distal to the thrombus may be oligemic- demonstrating a decreased number and caliber of vessels, and decreased attenuation. Because most emboli are longer than 1-2 mm, filling defects that are visible on only one 1.25 mm image, and not on contiguous images, are more likely to be artifacts than emboli [91].
Although no pleuroparenchymal findings are noted in about 30% of cases of acute PE [35], parenchymal abnormalities are commonly detected [33,35]. Findings include pulmonary hemorrhage, which appears as an area of ground-glass attenuation or air-space consolidation (indistinguishable from edema or pneumonia), and pulmonary infarction (occurs in 10% of cases). Infarction classically appears as a peripheral wedge-shaped, pleural-based opacification with its apex directed towards the hilum. Wedge shaped opacities can be found in 25-67% of patients with PE, but the finding can also occur in patients without embolism [33,35]. There may be a thickened/enlarged vessel identified entering at the apex of the opacification ("vascular sign") and this can help distinguish an infarct from an area of pneumonia [35,120]. Central air lucencies are another finding that suggests infarct, whereas the presence of air bronchograms is more suggestive of an infiltrate [120]. Infarcts may have a truncated apex if the lobules immediately subtended by the embolus have adequate collateral bronchial circulation. Hemorrhage without infarction usually resolves within a week, while infarcts decrease slowly in size over 3 to 5 weeks. The infarct may resolve completely, or leave a fibrous scar with associated pleural thickening. True cavitation is unusual with bland infarction and typically implies secondary infection of the necrotic tissue or septic emboli. Pleural effusions are a common feature of PE (roughly 50% of cases), but the finding is not specific as it can also be seen in patients without PE [33,35]. Effusions develop almost immediately, are usually small and unilateral, and are often hemorrhagic [3].
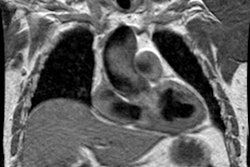
Right ventricular dysfunction as a result of PE is an independent predictor of an increased risk for mortality [109,150]. Findings which may suggest right ventricular strain or failure include right ventricular dilatation (in which the greatest short axis measurement of the right ventricular cavity is wider than the left ventricular cavity [103,106,190]), deviation of the interventricular septum toward the left ventricle, reflux of contrast into the IVC (although the usefulness of this finding diminishes with high injections rates of greater than 3 mL/sec [106]) [103], and a pulmonary embolism index of greater than 60% [80]. The RV diameter is typically measured on the transverse section that shows the tricuspid valve at its widest [113]. The diameter is measured at the widest point from the inner wall to the inner wall (i.e.- excluding the myocardium and typically this is the basal third of the RV) [113]. The LV is measured on the transverse image showing the mitral valve at its widest [113]. Some suggest that RV measurements obtained on a reconstructed 4-chamber view may be superior to those obtained on routine transaxial slices [109], but others have not found this to be true [171]. ECG synchronization imaging may help to further define RV function in PE patients through assessment of end-systolic volumes and RVEF measurement [109]. The greater the right-to-left ventricle ratio, the greater the risk of death [115]. A ratio of 1.0 is associated with a 5% chance of death; a ratio of 1.3 has a 10% chance of death, and a ratio of 2.3 up to 50% chance of death [115]. Unfortunately, a single CT scan does not reliably help to distinguish between an enlarged RV/LV diameter ratio secondary to acute PE and an enlarged ratio due to a pre-existing condition [129]. Therefore, if prior CT examinations are available, a change in the diameter ratio also carries important prognostic information [129]. For PE-related mortality- an interval increase of more than 18% in the diameter ratio has a significantly higher PPV than a diameter ratio of greater than 1.0 [129]. A PA diameter of greater than 30mm is indicative of a PA pressure of over 20 mm Hg [95]. Enlargement of the azygous vein greater than 10.4 mm may also be an indicator of right heart strain and increased mortality [103]. Other authors have also reported a reduction in the volume of the left atrium and decreased diameter of the pulmonary veins in patients with massive pulmonary embolism [137].
In one study, follow-up exams performed 6 weeks after acute
pulmonary embolism, complete resolution of thrombus is found in
only 32% of patients (most commonly in patients with low initial
clot burdens) [25]. The majority of patients demonstrate some
clot resolution and some abnormalities such as eccentric emboli
contiguous with the vessel wall or filling defects with central
contrast material representing recanalization
[25]. This is important to note, as these findings were
previously felt to represent changes of chronic thromboembolic disease [25]. However,
another study found that most patients (81%) showed complete
resolution of PE on CT angiography after 28 days [149] and in
another study, 77% of the clots resolved between 29-90 days, and
94% after 90 days [177]. One study reported rapid clearing of
pulmonary embolism (2-7 days) was noted for a large percentage
of PE's in the main and lobar pulmonary arteries [149]. However,
another study reported that clots in the peripheral vessels
resolved faster than clot in the central pulmonary arteries
[177]. Complete resolution of perfusion abnormalities has been
reported in 65-85% of patients on V/Q scanning after one year
[177].
Pitfalls and limitations in CT imaging for PE:
Inconclusive/Indeterminate exam: Technically inadequate exams have been reported in 1 to 12% of cases and a technically adequate exam may still be considered inconclusive in up to 9% of cases [9,68,83,86,92,115]. Common causes for indeterminate exams include motion/respiratory motion (74%), poor vascular enhancement (40%), parenchymal disease (12%), body habitus (7%), and streak artifacts (7%) [115]. In PIOPED, the rate of non-diagnostic pulmonary angiography was 3% [75]. In a small number of patients, adequate contrast enhancement may be present in the SVC, left-sided cardiac chambers, and aorta- yet opacification of the pulmonary arteries is inadequate [91]. This has the appearance of two separate contrast boluses with an intervening gap [91]. Postulated causes for this artifact include: 1- Right-to-left shunting across a patent foramen ovale caused by a Valsalva maneuver during breathhold [69]; or 2- A column of unopacified blood entering the right atrium from the IVC (due to deep inspiration) which transiently interrupts the contrast bolus [91]. Deep inspiration causes a decrease in intrathoracic pressure and a pressure gradient known as the thoracoabdominal pump [146,162]. The venous return to the right side of the heart increases by nearly 50% during deep inspiration and most of this venous return originates from the IVC [146].When CTA is performed using a deep inspiration he relative contribution of the IVC to the right heart can increase and can lead to interruption of the contrast bolus entering the right heart from the SVC [162]. A right to left shunt (patent foramen ovale) also results in a lower degree of pulmonary artery enhancement which can reduce exam quality [17].
Respiratory motion can result in a degree of limitation in exam interpretation in up to 27% of cases [54].
Despite an inconclusive result, anticoagulation is commonly withheld in these cases without adverse effects [86]. However, in one study of patients with initial inconclusive CT PE exams, a retrospective review indicated a 2.1% incidence of PE that was missed on initial interpretation [92]. In this same study, only 34% of patients with an initial indeterminate CT PE study underwent further evaluation [92]. Among this group, one was found to have PE at angiography and 4 were found to have DVT at US [92]. Therefore, further evaluation to exclude PE may be required in patients with indeterminate/inconclusive CT PE exams.
Obliquely/horizontally oriented vessels within the right middle lobe and left lingular region make detection of emboli in these sites difficult [23], but isolated emboli to these vessels would be rare (2.5% in one study [10]). Anterior segmental arteries of the upper lobes and the superior segmental arteries of the lower lobes also run obliquely. Angled 2D reformations through the obliquely oriented vessels may be useful in excluding PE [12], but may not be necessary with the use of smaller collimation (2mm) [17].
CT PE can be inaccurate in patients with a low pretest probability for PE [184]. False positive rates can be as high as 26-42%, and have been reported to be as high as 59.4% for solitary subsegmental PE [184]. Most false-positive findings occur in the lower lobes [184]. Intersegmental lymph nodes may be misinterpreted as clots- viewing images so that vessels are displayed along their long axis helps to decrease this artifact. Breathing artifact or cardiac motion/pulsation can result in false positive exams (ie: pseudoarterial filling defects) [9,26] and the most common cause of false-positive studies [184]. Review of lung parenchymal windows should reveal other evidence of respiratory motion which may not be evident on mediastinal window settings. Unilateral extensive airspace consolidation or atelectasis can result in ipsilateral increased vascular resistance with the resultant slow flow producing spurious filling defects within the pulmonary arteries that can be mistaken for emboli [17,37,80]. Patients with CHF may have a circumferential collar of low attenuation around a proximal segmental artery secondary to perivascular edema [17]. Beam-hardening streak artifacts from dense contrast material in the SVC can overlie the right upper lobe pulmonary arteries and may mimick PE [80]. The frequency of this artifact can be reduced by using a saline bolus immediately after the contrast injection [115].
Flow related central regions of decreased attenuation may be identified and mistaken for clot on studies performed during the terminal phases of contrast injection. This artifact is suspected to result from laminar flow of the contrast media. There is more rapid inflow of unopacified blood into the central portion of the vessel, while opacified blood persists peripherally due to slower flow near the vessel wall [24].
In patients that have undergone lung resection/pneumonectomy
surgery, in situ thrombus may form within the arterial stump (up
to 12% of patients [192]) [80]. This should not be mistaken for
a pulmonary embolism [80]. In situ thrombus has also been
described in primary pulmonary hypertension, sickle cell
disease, and congenital heart disease with Eidenmenger syndrome
[192]. Some authors suggest in situ thrombus can also occur in
association with radiation therapy when the pulmonary artery is
included in the radiation therapy volume [192].
For patients with renal dysfunction, prophylactic hydration
with sodium bicarbonate may help to decrease the incidence of
renal dysfunction following contrast administration [108]. An
isotonic solution of sodium bicarbonate 3mL/kg/hr for one hour
before and 6 hours after the administration of contrast material
has been recommended [108]. Nonsteroidal
antiinflammatory drugs and dipyridamole should be stopped as early
as possible before contrast administration [108]. Metformin should also be discontinued
before contrast administration [108]. Metformin
does not cause renal dysfunction,
however, should contrast induced nephropathy occur, metformin will accumulate in body
tissues and could cause a lactic acidosis [108]. Metformin may be resumed when renal
function is shown to be normal [108].
Overuse of pulmonary CTA has been associated with the detection
of clinically irrelevant incidental findings in up to 25% of
examination that obligate further workup[185].
Combined helical CT for exclusion of PE and DVT:
More than 90% of pulmonary emboli arise from the deep veins of the legs or pelvis [58]. DVT has been reported in 29-43% of patients with proven PE and can be found in 2.5-18% of patients suspected of having PE [131]. The use of helical CT for the exclusion of deep venous thrombosis during imaging for pulmonary embolism has been performed [20,21,39,40,51,54]. This type of combined CT PE/DVT exam allows for "one-stop" evaluation of patients with suspected pulmonary embolism. The CT examination can easily identify clot within the deep venous system of the lower extremities (DVT is found in about 9-10.5% of patients that undergo CT PE examinations [68,85]). CT can also identify clot within the pelvic veins or IVC which are not well assessed by ultrasound (the pelvic veins or IVC can be the source for pulmonary embolism in 3% to 11% of cases of PE [51,58,122]). CT venography results in a 1-38% increase in the diagnosis of thromboembolic disease over CTPA alone [85,105,122,124,127,131]. Unsuspected lower extremity DVT can be found in up to 6.8% of patients with an underlying malignancy [117]. In PIOPED II, the addition of CT venography increased the sensitivity for the detection of veno-embolic disease from 83% to 90% [132]. The PIOPED II investigators recommend that if CT is appropriate, CTA should be obtained in combination with CTV in most patients [124].
Venous enhancement persists for some time following the intravenous injection of contrast material which permits detection of clot within the venous structures [21]. Ideally, scanning should be performed during the period of peak venous enhancement. Venous enhancement increases slowly and has a gradual decline [40]. A time delay of approximately 120 seconds following completion of the CT PA exam allows for good venous enhancement in the majority of patients [40]- this is roughly 3 minutes following initiation of the contrast injection [57]. In general, only about 1-2% of examinations for DVT are inconclusive- usually due to poor venous enhancement or streak artifacts from orthopedic hardware or dense arterial calcifications [51,56], however, up to 16% of exams may have segments of the deep venous system which cannot be accurately assessed (ie: due to beam hardening artifacts associated with orthopedic hardware, etc.) [56]. Less than optimal quality exams are more commonly seen in very obese patients (BMI > 35)- up to 34% of exams can be of poor quality [124]. Scattered radiation producing a poor signal to noise ratio is the most likely cause for decreased diagnostic accuracy in very obese patients [124]. NOTE: Acute thrombi less than 8 days old can have an average attenuation value of as high as 66HU [40]. Thrombi older than 8 days have an average attenuation of 55 HU [40]. Intravenous enhancement should optimally exceed these levels for clot detection with optimum venous enhancement of more than 80 HU [116].
No standard protocol has been established for this procedure.
One protocol calls for axial 5 mm scans performed at 4 cm
intervals from the upper calves to the diaphragm using a kVp of 120 and an mAs
of 250 [39,58,68]. The average radiation dose from this type of
DVT exam is 12 mGy for the abdomen
and 19 mGy for the legs [68,122]
(however, other authors quote much lower exposures for the legs-
0.6 mSv [131]). Although most leg
clots are long segment, a 4 cm gap between slices will obviously
result in the failure to identify all cases of deep venous
thrombosis [58,85]. In studies
determining DVT length, between 94-98% are
longer than 2 cm- meaning 2-6% of DVT's could be missed if using
a slice gap of 2 cm or more [118,122,141]. A slice gap of 15 mm
should be adequate to detect most DVT's .
However, some authors have concluded that using a slice gap can
potentially lead to false negative findings on CT or an
underestimation of the extent of clot [56,85,141].
Lowering the kVp setting for CTV has been suggested in order to
further reduce patient radiation exposure [160]. A lower kVp
results in improved vascular enhancement, however, image noise
is also increased, but this does not appear to affect image
interpretation [160].
Helical acquisitions using 1 cm images (pitch of 1) performed from the iliac wings to the tibial plateau may also be performed [21,51,85]. If using this continuous helical acquisition, one must consider the large quantity of images generated and the additional radiation dose to the patient (roughly 22 mGy for the abdomen [68] and gonadal doses of 2.1 to 10.7 mSv [85]). The gonadal dose is two or more magnitudes larger than for CT pulmonary angiography alone [122]. Another article quotes the ovarian dose to be increased by 500 times and the testicular dose increased by 2000 times when CTV is performed [131]. Dose modulation can be useful in reducing the radiation dose by 35-60% [105]. Because up to 90% of the absorbed dose is the result of radiation exposure to the pelvis [131], the dose can also be reduced by omitting the iliac veins and IVC (i.e.: begin the acquisition at the acetabulum), however, the iliac veins or IVC may show thrombi in up to 3% of patients with no evidence of clot in the femoral or popliteal veins [108,118]. However, in PIOPED II, all patients with isolated IVC or iliac vein clot had PE detected on CT angiography [122]. In another study of 2074 CT PE exams, isolated pelvic clot was found in only 0.1% of cases [131]. This article concluded that CT venography of the pelvis does not improve detection of venous thromboembolic disease and should be omitted from the CT DVT exam [131]. The pelvis might best be imaged in patients with specific risk factors for pelvic thrombi such as pelvic surgery or post-partum patients [124,131]. Using a slice gap technique (5 mm slices every 2 cm) and starting at the acetabulum with automated tube current modulation can reduce the radiation dose by about 75% to about 0.6-2.3 mSv [122,124,140]. In some patients- such as young women, sonography might be a preferable option to CTV provided that its diagnostic accuracy would be similar to CTV [124].
Indirect CT venography can identify isolated DVT in 3.4% to 5% of patients with a negative CT PE exam [51,58,83,131,141]. In PIOPED II, 8% of patients referred for evaluation had DVT only [122]. The finding of DVT in these patients obviously results in a change in patient management. However, the likelihood for isolated DVT may be increased only in certain high risk patients (underlying malignancy, prior thromboembolic event, recent surgery, severely ill-patients, and ICU patients) and the incidence has been shown to be much lower (0.72%) in low risk patients [131,140]. In the PIOPED II study, CT venography was positive for DVT in 60% of patients with signs and/or symptoms of DVT versus only 8% of patients without them [140]. The sensitivity and specificity of helical CT for the detection of femoral popliteal DVT is comparable to lower extremity US [51,52]- sensitivity 89-97%, specificity 94-100%, and accuracy 93% [54,55,58]. There are cases in which the CT exam of the lower extremities may be positive and the US exam negative (excluding pelvic vessels and the IVC)- possibly due to flow artifacts or volume averaging with valves [51,52,54]. False positive CT exams typically involve a subtle finding seen on only a single image [54]. It has been recommended that US be used to confirm isloated DVT identified on CT prior to initiation of anticoagulation [54]. Of course, because there is always a time delay between the two exams, one can never be absolutely certain that these cases don't actually represent false negative ultrasounds [51]. Extensive, bilateral lower extremity thrombosis may also not be properly identified at CT [54]. In cases of bilateral, extensive DVT findings which should suggest the diagnosis include prolonged arterial phase enhancement, venous dilatation, and vessel wall enhancement [54]. Flow artifacts can be reduced in patients with suspected abnormal hemodynamic status by increasing the delay prior to the DVT portion of the exam to 4 minutes post injection [52]. Up to 10.8% of exams can be nondiagnostic [141]. Interobserver disagreement can occur in up to 12% of cases [56].
CT PA and Pregnancy/Puerperium
period:
Pregnancy is associated with a 5 to 10-fold increase in the
prevalence of venous thromboembolism
and pulmonary embolism is a leading cause of maternal death
[142,166]. The incidence of pregnancy associated PE is about
2-12/10,000 pregnancies [166]. The incidence of
venous-thromboembolism is similar in all three trimesters, and
the majority of cases occur in the post-partum period [166].
Despite the high risk, a recent study found that PE was
diagnosed in only 3.7% of pregnant patients who underwent CT PE
imaging [157]. Pregnancy-associated DVT is three times more
common than pregnancy associated PE, and pregnant patients are
75-96% more likely to have DVT in the left leg compared to the
right (likely due to compression of the left iliac vein by the
right iliac artery and the gravid uterus [166]. Pregnant
patients are also nearly three times more likely to have
isolated DVT in the pelvic veins- and may present with abdominal
pain rather than lower extremity pain or swelling [166]. The
puerperium period is defined as the 6-week period after delivery
and is associated with an even higher rate of thrombosis- with
up to 50% of pregnancy related episodes of PE occuring during
this period [178].
Unfortunately, the D-dimer assay has a limited role in pregnancy as levels rise as pregnancy progresses producing false-positive results [142]. None-the-less, about 50% of pregnant patients will have a normal D-dimer value, and the test still maintains a high negative predictive value even during pregnancy [157]. Unfortunately, PE can still occur in patients with negative D-dimer levels [142,164,166]. The Fleischner Society recommends that lower extremity US should be the first imaging test used in pregnant patients with suspected PE- if the exam is positive- no additional imaging is required [122]. Other guidelines by the American Thoracic Society recommend lower extremity US only if there are clinical signs of symptoms of DVT [164]. A unique predisposition for DVT of the left lower extremity (up to 75-96% of cases) has been shown in pregnant patients and is thought to be related to compression of the left common iliac vein by the crossing right iliac artery or to increased mass effect by the gravid uterus [152]. It has been suggested that lower extremity US imaging will detect DVT in 13-15% of pregnant patients with suspected PE [108].
Unfortunately, the non-diagnostic rate of CT PA is higher in pregnant patients (19% to 36% of cases [146,155,162]) due to increased circulatory volume/hemodilution (blood volume increases by 50% during early pregnancy), an altered (increased) cardiac output/hyperdynamic state that increases flow artifacts, and interruption of the contrast flow by unopacified blood from the IVC (IVC pressure is particularly high when the pregnant patient is supine) [142,146,155,162]. The rate of diagnostic inadequate VQ scans in pregnant patients is much lower (4%) [157]. Definitive results can be obtained from the VQ scan in 94-96% of patients with normal CXR's [164]. An article comparing the accuracy of CTPA and V/Q scanning in pregnant patients found fewer non-diagnostic V/Q studies (2.1% versus 35.7%) (in this study, patients with CXR abnormalities underwent CTPA imaging and not V/Q scanning) [146]. However, in another study, the rate of indeterminant exams was comparable between CT PE and VQ imaging (19% for both modalities) [155]. Unlike VQ scanning, CT PE imaging can also suggest an alternative diagnosis producing the patients symptoms [157]
However, optimizing the CTPA protocol for pregnancy may help to reduce the number of non-diagnostic studies [162]. Using high iodine concentration IV contrast (350-370), a high rate of contrast injection (6cc/sec), a high volume of contrast material (95cc), and a shallow inspiration without valsalva has been shown to reduce the number of non-diagnostic exams [162,164].
In a survey of thoracic radiologists, about 75% of institutions
perform CTPA exams on pregnant patients [72], however, a
majority (69%) of PIOPED II investigators recommend V/Q scanning
(up to 73-92% of V/Q scans in pregnant patients can demonstrate
normal findings [142]) [108]. Additionally, the American
Thoracic Society and Society of Thoracic Radiology recommend VQ
imaging as the first imaging test to exclude PE if the patient
has a normal CXR (CT PE imaging is recommended when the CXR is
abnormal or if the VQ scan is nondiagnostic) [164].
Because there is no direct radiation to the fetus, the mean fetal radiation dose from CTPA imaging is lower than from VQ scanning [157]. Fetal dose has been estimated to be 240-660 uGy [72,98]. The fetal dose, however, varies with the patients trimester- approximately 3.3-20.2 μGy during the first trimester, 7.9-76.6 μGy for the second trimester, and 51.3-130.8 μGy for the third trimester [157]. This is lower than the fetal dose from V/Q scanning [107]. However, the fetal radiation dose from V/Q scanning is still very low (0.25-0.36 mGy at 0 months and 0.31-0.32 mGy at 3 months [108]; other authors quote exposure of 100-370 μGy for the V/Q exam during all trimesters [157]) and well within safety guidelines (see separate discussion). Oral barium and lead shielding can also be used to decrease fetal exposure during CT PE imaging [142].
Drawbacks of the CTPA exam which must be considered include:
1- The greatly increased maternal radiation exposure (2.2-7.3 mSv versus 0.9-1.4 mSv for the V/Q exam)
2- Increased radiation exposure to the breasts with subsequent increased risk for breast cancer from the CTPA exam (effective dose of 10-70 mGy for CTA versus 0.22-0.28 mGy for the perfusion lung scan and 0.11 to 0.3 mGy for low dose perfusion scintigraphy [108,155,166]). The lifetime relative risk of radiation-induced breast and lung cancer in a 25 year old woman who undergoes a single CT PE study is 1.011 and 1.022, respectively [164].
3- The small risk of a maternal reaction to the contrast media
4- Potential fetal side effects from IV contrast [72,107]. Both iodinated and gadolinium contrast agents cross the placenta and enter the fetal circulation and amniotic fluid [18]. Elevated iodine levels can be detected in amniotic fluid following a single or repeat exposure to iodinated products [153]. Free iodine in contrast media has the potential to depress fetal and neonatal thyroid function (neonatal hypothyroidism) [125]. Consequently, if iodinated contrast medium is administered during pregnancy, neonatal thyroid function should be checked during the first week after delivery [122,125]. To date, there have been no controlled studies in humans to assess the effects of IV contrast on the fetus [107]. A retrospective review of 343 pregnant patients that had undergone CTPE imaging found that only one newborn in the cohort had a transiently abnormal elevated TSH level at birth, which normalized by day 6 of life [153]. These authors concluded that a single, high-dose in utero exposure to water-soluable low-osmolar iodinated intravenous contrast is unlikely to have a clinically important effect on thyroid function at birth [153]. However, it should be noted that 25% of the newborns in the study had TSH levels drawn because they had T4 values that, although in normal limits, were in the lowest 10th percentile or for clinical purposes unrelated to screening [153].
Additionally, incidental findings detected on the CTPA study can lead to additional imaging and increased patient radiation exposure. Patients should be informed of the risks of the procedure and informed consent obtained [72,107].
Non-contrast CT: Rarely, central pulmonary emboli can be seen on unenhanced CT imaging of the chest [71]. The clot will appear as high attenuation within the pulmonary arteries [71]. The clot is more likely to be identified in patients with subnormal hematocrits [71].
MRI:
On MRI, using standard or gated spin echo examinations, pulmonary emboli appear as foci of increased signal intensity within the signal void of the pulmonary artery lumen. Occasionally, blood within the vessels may demonstrate high signal- usually this occurs during the diastolic portion of the gated exam. By obtaining multiple acquisitions gated to varying points in the cardiac cycle (each 100 msecapart) a sequence of images can be obtained and the increased signal associated with slow flow will disappear on at least one image. Patients with pulmonary hypertension, however, may have markedly diminished flow velocities and even during systole the increased signal within the vessel may remain. This finding was observed in all patients with pulmonary systolic pressures greater than 80 mm Hg [14].
It is now more common to evaluate the pulmonary circulation
using MR angiography during suspended respiration following the
intravenous administration of gadolinium. MR has been shown to
have a sensitivity between 77-100%,
and a specificity of 89-98% [30,101,122]. However, in the PIOPED
III study, up to 25% of patients had technically inadequate
exams [157]. MR can best detect central and lobar PE
(sensitivity 79-100% [157]) and has reasonable sensitivity for
segmental emboli (50-84% [122,157]), but is not adequate for the
detection of subsegmental emboli
(sensitivity decreases to 40-55% or lower) [30,101,122,157] and
is also less sensitive for the detection of emboli in the
lingula [167]. In a small number of patients, a study comparing
various MR sequences for the detection of PE that had been
documented on CT PE imaging found a sensitivity of 55% for MR
pulmonary angiography (14% of the MR angiography exams were
technically inadequate secondary to motion), 67% for triggered
true FISP, and 73% for 3D GRE [167]. Combining all three
sequences improved sensitivity to 84% and decreased the number
of technically inadequate exams [167].
"One stop" MR imaging which includes the lower extremities for DVT has also been performed [101]. About 17% of patients will have a DVT revealed on MR venography in the absence of a detectable pulmonary embolism [101].
Transthoracic/Transesophageal
Echocardiography:
The one advantage of these studies is that they can be performed at the patients bedside, however, in comparison to helical CT, transthoracic and transesophgeal echo have limited accuracy for detecting pulmonary embolism. The central pulmonary arteries can be visualized by transesophageal echo and in one study, the sensitivity for central PE was 82%. Unfortunately, in order to detect more peripheral PE these procedures rely on indirect evidence of PE including tricuspid regurge, RV dilatation, paradoxical wall motion, and widening of the pulmonary artery diameter. Overall sensitivity in one study was 59%, with a 77% overall specificity [15]. Right ventricular hypokinesis detected by echocardiography is an important predictor of mortality associated with acute pulmonary embolism [50].
REFERENCES:
(1) Radiology 1996; Diagnosis of acute pulmonary embolism: time for a new approach 199 (1): 25-27 (No abstract available)
(2) Radiol Clin North Am 1993; 31(4): 849-858
(4) Radiology 1993;189:133-136
(5) Am Rev Resp Dis 1978; 177: 829-834
(6) RadioGraphics 1997; CT of acute pulmonary emboli: where does it fit? 17: 1037-1042 (No abstract available)
(7) Radiology 1978; 126:561-567
(8) Radiology 1997; 204: 157-163
(9) Radiology 1996; 200: 699-706
(10) Radiology 1996; 199: 31-35
(13) Radiology 1997; Pulmonary arteries must be seen before they can be assessed.204: 11-12 (No abstract available)
(14) AJR 1987; 149: 15-21
(16) J Thorac Image 1997; Reply to opinions.12: 100-102 (No abstract available)
(17) J Thorac Image 1997; 12: 103-117
(18) AJR 1997; CT Evaluation of pulmonary embolism: Technique and interpretation. 169: 959-965 (No abstract available)
(19) Radiology 1997; 205: 453-458
(20) AJR 1998; Loud PA, et al. Combined CT venography and pulmonary angiography: A new diagnostic technique for suspected thromboembolic disease. 170: 951-954 (No abstract available)
(21) ARRS 98th Annual Meeting Abstract Book 1998; Yankelevitz DF, et al. Technical considerations for combining pulmonary CT angiography with lower extremity CT venography. 63-63
(22) Radiology 1998. 207: 753-758
(23) Radiology 1998; 208: 201-208
(24) Radiology 1998; 208: 209-215
(25) J Comput Assist Tomogr 1998; Van Rossum AB, et al. Spiral CT appearance of resolving clots at 6 week follow-up after acute pulmonary embolism. 23 (3): 413-417
(26) AJR 1998; Beigelman C, et al. Pitfalls in diagnosis of pulmonary embolism with helical CT angiography. 171: 579-585 (No abstract available)
(27) Radiology 1998; Drucker EA, et al. Acute pulmonary embolism: Assessment of helical CT for diagnosis. 209: 235-241
(28) AJR 1998; Diffin DC, et al. Effect of anatomic distribution of pulmonary emboli on interobserver agreement in the interpretation of pulmonary angiography. 171: 1085-1089
(29) AJR 1999; Chartrand-Lefebvre C, et al. Contrast-enhanced helical CT for pulmonary embolism detection: inter- and Intraobserver agreement among radiologists with variable experience. 172: 107-112.
(30) Radiology 1999; Gupta A, et al. Acute pulmonary embolism: Diagnosis with MR angiography. 210: 353-359
(31) Radiology 1999; Stein PD, et al. Reassessment of pulmonary angiography for the diagnosis of pulmonary embolism: Relation of interpreter agreement to the order of the involved pulmonary arterial branch. 210: 689-691
(32) Radiology 1999; Kim KI, et al. Clinically suspected pulmonary embolism: Utility of spiral CT. 210: 693-697
(33) Radiology 1999; Shah AA, et al. Parenchymal and pleural findings in patients with and patients without acute pulmonary embolism detected at spiral CT. 211: 147-153
(34) AJR 1999; Garg K, et al. Clinical validity of helical CT being interpreted as negative for pulmonary embolism: Implications for patient treatment. 172: 1627-1631
(35) J Comput Assist Tomogr 1999; Johnson PT, et al. Spiral CT of acute pulmonary thromboembolism: Evaluation of pleuroparenchymal abnormalities. 23 (3): 369-373
(36) Chest 1999; Arcasoy SM, Kreit JW. Thrombolytic therapy of pulmonary embolism. A comprehensive review of current evidence. 115: 1695-1707
(37) Radiology 1999; Remy-Jardin M, Remy J. Spiral CT angiography of the pulmonary circulation. 212: 615-636
(38) Radiology 1999; Novelline RA, et al. Helical CT in emergency radiology. 213: 321-339
(39) AJR 2000; Loud PA, et al. Combined CT venography and pulmonary angiography in suspected thromboembolic disease: Diagnostic accuracy for deep venous evasluation. 174: 61-65
(40) AJR 2000; Yankelevitz DF, et al. Optimization of combined CT pulmonary angiography with lower extremity CT venography. 174: 67-69
(41) Ann Intern Med 2000; Rathbun SW, et al. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. 132: 227-232
(42) Radiology 2000; Schoepf UJ, et al. Segmental and subsegmental pulmonary arteries: Evaluation with electron-beam versus spiral CT. 214: 433-439
(43) J Vasc Interv Radiol 1999; Lomis NN, et al. Clinical outcomes of patients after a negative spiral CT pulmonary arteriogram in the evaluation of acute pulmonary embolism. 10: 707-712
(44) AJR 2000; Blachere H, et al. Pulmonary embolism revealed on helical CT angiography: Comparison with ventilation-perfusion radionuclide lung scanning. 174: 1041-1047
(45) Radiology 2000; de Monye W, et al. Suspected pulmonary embolism: Prevalence and anatomic distribution in 487 consecutive patients. 215: 184-188
(46) Radiology 2000; Goodman LR, et al. Subsequent pulmonary embolism: Risk after a negative helical CT pulmonary angiogram- prospective comparison with scintigraphy. 215: 535-542
(47) AJR 2000; Smith TP. Pulmonary embolism: What's wrong with this diagnosis? 174: 1489-1497 (Review. No abstract available)
(48) Radiology 2000; Athanasoulis CA, et al. Inferior vena caval filters: Review of a 26-year single center clinical experience. 216: 54-66
(49) AJR 2000; Remy-Jardin M, et al. Clinical value of thin collimation in the diagnostic workup of pulmonary embolism. 175: 407-411
(50) Chest 2000; Elliott CG, et al. Chest radiographs in acute pulmonary embolism. Results from the international cooperative pulmonary embolism registry. 118: 33-38
(51) Radiology 2000; Cham MD, et al. Deep venous thrombosis: Detection by using indirect CT venography. 216: 744-751
(52) AJR 2000; Garg K, et al. Thromboembolic disease: Comparison of combined CT pulmonary angiography and venography with bilateral leg sonography in 70 patients. 175: 997-1001
(53) Radiology 2000; Qanadli SD, et al. Pulmonary embolism detection: Prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. 217: 447-455
(54) AJR 2000; Duwe KM, et al. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: A retrospective comparison of helical CT venography and sonography. 175: 1525-1531
(55) AJR 2001; Coche EE, et al. Using dual-detector helical CT angiography to detect deep venous thrombosis in patients with suspicion of pulmonary embolism: diagnostic value and additional findings. 176: 1035-1039
(56) AJR 2001; Garg K, et al. Thromboembolic disease: Variability of interobserver agreement in the interpretation of CT venography with CT pulmonary angiography. 176: 1043-1047
(57) AJR 2001; Bruce D, et al. Combined CT venography and pulmonary angiography: How much venous enhancement is routinely obtained? 176: 1281-1285
(58) Radiology 2001; Loud PA, et al. Deep venous thrombosis with suspected pulmonary embolism: Detection with combined CT venography and pulmonary angiography. 219: 498-502
(59) Radiology 2001; Ghaye B, et al. Peripheral pulmonary arteries: How far in the lung does multi-detector row spiral CT allow analysis? 219: 629-636
(60) Arch Surg 2001; Velmahos GC, et al. Spiral computed tomography for the diagnosis of pulmonary embolism in critically ill surgical patients. A comparison with pulmonary angiography. 136: 505-510
(61) Arch Intern Med 2001; Goldstein NM, et al. The impact of the introduction of a rapid D-dimer assay on the diagnostic evaluation of suspected pulmonary embolism. 161: 567-571
(62) JAMA 2001; Kline JA, et al. Diagnostic accuracy of a bedside D-dimer assay and alveolar dead-space measurement for rapid exclusion of pulmonary embolism. A mutlicenter study. 285: 761-768
(63) Radiology 2001; Raptopoulos V, Boiselle PM. Multi-detector row spiral CT pulmonary angiography: comparison with single-detector row spiral CT. 221: 606-613
(64) Prog Cardiovasc Dis. 1994; Stein PD, Gottschalk A. Critical review of ventilation/perfusion lung scans in acute pulmonary embolism. 37: 13-24 (No abstract available)
(65) Radiology 2002; Schoepf UJ, et al. Subsegmental pulmonary emboli: improved detection with thin-collimation multi-detector row spiral CT. 222: 483-490
(66) Radiology 2002; Tillie-Leblond I, et al. Risk of pulmonary embolism after negative spiral CT angiogram in patients with pulmonary disease: 1-year clinical follow-up study. 223: 461-467
(67) AJR 2002; Ginsberg MS, et al. Clinical usefulness of imaging performed after CT angiography that was negative for pulmonary embolus in a high-risk oncologic population. 179: 1205-1208
(68) Radiographics 2002; Katz DS, et al. Combined CT venography and pulmonary angiography: a comprehensive review. 22: S3-S24
(69) Radiology 2003; Henk CB, et al. Suspected pulmonary embolism: enhancement of pulmonary arteries at deep-inspiration CT angiography- influence of patent foramen ovale and atrial-septal defect. 226: 749-755
(70) Radiology 2003; Patel S, et al. Pulmonary embolism: optimization of small pulmonary artery visualization at multi-detector row CT. 227: 455-460
(71) AJR 2003; Kanne JP, et al. Six cases of acute central pulmonary embolism revealed on unenhaced multidetector CT of the chest. 180: 1661-1664
(72) AJR 2003; Schuster ME, et al. Pulmonary embolism in pregnant patients: a survey of practices and polices for CT pulmonary angiography. 181: 1495-1498
(73) Radiology 2003; Coche E, et al. Diagnosis of acute pulmonary embolism in outpatients: comparison of thin-collimation multi-detector row spiral CT and planar ventilation-perfusion scintigraphy. 229: 757-765
(74) Radiology 2004; Schoepf UJ, Costello P. CT angiography for diagnosis of pulmonary embolism: state of the art. 230: 329-337
(75) AJR 2004; Kavanagh EC, et al. Risk of pulmonary embolism after negative MDCT pulmonary angiography findings. 182: 499-504
(76) AJR 2004; Abcarian PW, et al. Role of a quantitative D-dimer assay in determining the need for CT angiography of acute pulmonary embolism. 182: 1377-1381
(77) Radiographics 2003; Han D, et al. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. 23: 1521-1539
(78) AJR 2004; Prologo JD, et al. C pulmonary angiography: a comparitive analysis of the utilization patterns in emergency departments and hospitalized patients between 1998 and 2003. 183: 1093-1096
(79) AJR 2004; Choe DH, et al. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. 183: 1097-1102
(80) Radiographics 2004; Wittram C, et al. CT angiography of pulmonary embolism: diagnostic criteroa and causes of misdiagnosis. 24: 1219-1238
(81) AJR 2004; Eng J, et al. Accuracy of CT in the diagnosis of pulmonary embolism: a systemic literature review. 183: 1819-1827
(82) Radiology 2004; Winer-Muram HT, et al. Suspected acute pulmonary embolism: evaluation with multidetector CT versus digital subtraction pulmonary arteriography. 233: 806-815
(83) Radiology 2004; Revel MP, et al. Diagnosing pulmonary embolism with four-detector row helical CT: prospective evaluation of 216 outpatients and inpatients. 234: 265-273
(84) AJR 2005; Storto ML, et al. Incidental detection of pulmonary embolism on routine MDCT of the chest. 184: 264-267
(85) Radiology 2005; Cham MD, et al. Thromboembolic disease detection at indirect CT venography versus CT pulmonary angiography. 234: 591-594
(86) AJR 2005; Eyer BA, et al. Clinician's response to radiologists' reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. 184: 623-628
(87) Radiology 2005; Goodman LR. Small pulmonary emboli: what do we know? 234: 654-658
(88) Radiology 2005; Hayashino Y, et al. Ventilation-perfusion scanning and helical CT in suspected pulmonary embolism: meta-analysis of diagnostic performance. 234: 740-748
(89) AJR 2005; Prologo JD, et al. The effect of single-detector CT versus MDCT on clinical outcomes in patients with suspected acute pulmonary embolism and negative results on CT pulmonary angiography. 184: 1231-1234
(90) AJR 2005; Patel S, Kazerooni EA. Helical CT for the evaluation of acute pulmonary embolism. 185: 135-149
(91) Radiol Clin N Am 2005; Chiles C, Carr JJ. Vascular diseases of the thorax: evaluation with multidetector CT. 43: 543-569
(92) Radiology 2005; Jones SE, Wittram C. The indeterminate CT pulmonary arteriogram: imaging characteristics and patient clinical outcome. 237: 329-337
(93) AJR 2005; Parker MS, et al. Female breast radiation exposure during CT pulmonary angiography. 185: 1228-1233
(94) JAMA 2006; Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. 295: 172-179
(95) Radiographics 2006; Ghaye B, et al. Can CT pulmonary angiography allow assessment of severity and prognosis in patients presenting with pulmonary embolism? What the radiologist needs to know. 26: 23-40
(96) Radiographics 2006; Restrepo CS, et al. Cardiovascular complications of human immunodeficiency virus infection. 26: 213-231
(97) AJR 2006; Subramanian RM, et al. Importance of pretest probability score and D-dimer assay before sonography for lower limb deep venous thrombosis. 186: 206-212
(98) AJR 2006; Hurwitz LM, et al. Radiation dose to the fetus from body MDCT during early gestation. 186: 871-876
(99) Radiology 2006; Remy-Jardin M, et al. Multi-detector row CT angiography of pulmonary circulation with gadolinium-based contrast agents: prospective evaluation in 60 patients. 238: 1022-1035
(100) Radiology 2006; Engelke C, et al. Pulmonary embolism at multidetector row CT of the chest: one-year survival of treated and untreated patients. 239: 563-575
(101) AJR 2006; Kluge A, et al. Experience in 207 combined MRI examinations for acute pulmonary embolism and deep vein thrombosis. 186: 1686-1696
(102) AJR 2006; Hurwitz LM, et al. Radiation dose to the female breast from 16-MDC body protocols. 186: 1718-1722
(103) Radiology 2006; Ghaye B, et al. Severe pulmonary embolism: pulmonary artery clot load scores and cardiovascular predictors of mortality. 239: 884-891
(104) Radiology 2006; Gladish GW, et al. Incidental pulmonary emboli in oncologic patients: prevalence, CT evaluation, and natural history. 240: 246-255
(105) Radiology 2006; Ghaye B, et al. Does mutli-detector row CT pulmonary angiography reduce the incremental value of indirect CT venography compared with single-detector row CT pulmonary angiography? 240: 256-262
(106) Radiographics 2006; Castaner E, et al. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. 26: 349-371
(107) Radiology 2006; Groves AM, et al. CT pulmonary angiography versus ventilation-perfusion scintigraphy in pregnancy: implications from a UK survey of doctors' knowledge of radiation exposure. 240: 765-770
(108) Radiology 2007; Stein PD, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. 242: 15-21
(109) Radiology 2007; Dogan H, et al. Right ventricular function in patients with acute pulmonary embolism: analysis with electrocardiography-synchronized multi-detector row CT. 242: 78-84
(110) Radiology 2007; Schueller-Weidekamm C, et al. CT angiography of pulmonary arteries to detect pulmonary embolism: improvement of vascular enhancement with low kilovoltage settings. 241: 899-907
(111) AJR 2007; Lee CH, et al. Determination of optimal timing window for pulmonary artery MDCT angiography. 188: 313-317
(112) N Engl J Med 2006; Stein PD, et al. Multidetector computed tomography for acute pulmonary embolism. 354: 2317-2327
(113) Radiology 2007; Araoz PA, et al. Pulmonary embolism: prognostic CT findings. 242: 889-897
(114) Radiology 2007; Bae KT, et al. Effect of patient weight and scanning duration on contrast enhancement during pulmonary multidetector CT angiography. 242: 582-589
(115) AJR 2007; Wittram C. How I do it: CT pulmonary angiography. 188: 1255-1261
(116) AJR 2007; Arakawa H, et al. CT pulmonary angiography and CT venography: factors associated with vessel enhancement. 189: 156-161
(117) AJR 2007; Cronin CG, et al. Prevalence and significance of asymptomatic venous thromboembolic disease found on oncologic staging CT. 189: 162-170
(118) AJR 2007; Goodman LR, et al. CT venography for deep venous thrombosis: continuous images versus reformatted discontinuous images using PIOPED II data. 189: 409-412
(119) J Nucl Med 2007; Strashun AM. A reduced role of V/Q scintigraphy in the diagnosis of acute pulmonary embolism. 48: 1405-1407
(120) Radiology 2007; Revel MP, et al. Is it possible to recognize pulmonary infarction on multisection CT images? 244: 875-882
(121) Radiology 2007; Wittram C, et al. Discordance between CT and angiography in the PIOPED II study. 244: 883-889
(122) Radiology 2007; Remy-Jardin M, et al. Management of suspected acute pulmonary embolism in the era of CT angiography: a statement fro the Fleischner society 245: 315-329
(123) Radiology 2007; Heyer CM, et al. Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. 245: 577-583
(124) AJR 2007; Goodman LR, et al. CT venography and commpression sonography are diagnostically equivalent: data from PIOPED II. 189: 1071-1076
(125) Radiographics 2007; Patel SJ, et al. Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. 27: 1705-1722
(126) Radiology 2007; Hurwitz LM, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. 245: 742-750
(127) JAMA 2007; Anderson DR, et al. Computed tomography pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism. A randomized controlled trial. 298: 2743-2753
(128) AJR 2007; MacKenzie JD, et al. Reduced-dose CT: effect on reader evaluation in detection of pulmonary embolism. 189: 1371-1379
(129) Radiology 2008; Lu MT, et al. Interval increase in right-left ventricular diameter ratios at CT as a predictor of 30-day mortality after acute pulmonary embolism: initial experience. 246: 281-287
(130) J Nucl Med 2008; Freeman LM. Don't bury the V/Q scan: it's as good as multidetector CT angiograms with a lot less radiation exposure. 49: 5-8
(131) AJR 2008; Hunsaker AR, et al. Routine pelvic and lower extremity CT venography in patients undergoing pulmonary CT angiography. 190: 322-326
(132) Radiology 2008; Sostman HD, et al. Acute pulmonary embolism: sensivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. 246: 941-946
(133) Radiology 2008; King V, et al. D-dimer assay to exclude pulmonary embolism in high-risk oncologic population: correlation with CT pulmonary angiography in an urgent care setting. 247: 854-861
(134) AJR 2008; Costantino MM, et al. CT angiography in the evaluation of acute pulmonary embolus. 191: 471-474
(135) J Nucl Med 2008; Stabin MG. Radiopharmaceuticals for nuclear cardiology: radiation dosimetry, uncertainties, and risk. 49: 1555-1563
(136) AJR 2008; Bierry G, et al. Venous thromboembolism and occult malignancy: simultaneous detection during pulmonary CT angiography with CT venography. 191: 885-889
(137) AJR 2008; Ocak I, Fuhrman C. CT angography findings of the left atrium and right ventricle in patients with massive pulmonary embolism. 191: 1072-1076
(138) Chest 2008; GIbson NS< et al. The importance of clinical probability assessment in interpreting a normal d-dimer in patients with suspected pulmonary embolism. 134: 789-793
(139) J Nucl Med 2008; Sostman HD, et al. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. 49: 1741-1748
(140) Radiology 2009; Goodman LR, et al. CT venography: a necessary adjunct to CT pulmonary angiography of a waste of time, money, and radiation? 250: 327-330
(141) AJR 2009; Nazaroglu H, et al. 64-MDCT pulmonary angiography and CT venography in the diagnosis of thromboembolic disease. 192: 654-661
(142) Radiographics 2009; Pahade J, et al. Imaging pregnant patients with suspected pulmonary embolism: what the radiologist needs to know. 29: 639-654
(143) AJR 2009; Matsuoka S, et al. Vascular enhancement and image quality of MDCT pulmonary angiography in 400 cases: comparison of standard and low kilovoltage settings. 192: 1651-1656
(144) AJR 2009; McCollough CH, et al. In defense of body CT. 193: 28-39
(145) AJR 2009; Gupta RT, et al. d-dimer and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. 193: 425-430
(146) AJR 2009; Ridge CA, et al. Pulmonary embolism in pregnancy: comparison of pulmonary CT angiography and lung scintigraphy. 193: 1223-1227
(147) AJR 2010; Stein EG, et al. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. 194: 392-397
(148) AJR 2010; Bhargavan M, et al. Frequency of use of imaging tests in the diagnosis of pulmonary embolism: effects of physician specialty, patient characteristics, and region. 194: 1018-1026
(149) AJR 2010; Stein PD, et al. Resolution of pulmonary embolism on CT pulmonary angiography. 194: 1263-1268
(150) AJR 2010; Kang DK, et al. Reproducibility of CT signs of right ventricular dysfunction in acute pulmonary embolism. 194: 1500-1506
(151) Radiology 2010; Pistolesi M. Pulmonary CT angiography in patients suspected of having pulmonary embolism: case finding or screening procedure? 256: 334-337
(152) Radiology 2010; Mamlouk MD, et al. Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. 256: 625-632
(153) Radiology 2010; Bourjeily G, et al. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. 256: 744-750
(154) AJR 2010; Shahir K, et al. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. 195: 669
(155) Radiology 2011; Revel MP, et al. Pulmonary embolism during pregnancy: diagnosis with lung scintigraphy or CT angiography? 258: 590-598
(156) J Cardiovasc Comput Tomogr 2011; Henzler T, et al. CT imaging of acute pulmonary embolism. 5: 3-11
(157) AJR 2011; Sadigh G, et al. Challenges, controversies, and hot topics in pulmonary embolism imaging. 196: 497-515
(158) AJR 2011; Coackley FV, et al. CT radiation dose: what can you do right now in your practice? 196: 619-625
(159) AJR 2011; Soo Hoo GW, et al. Does a clinical decision
rule using d-dimer level improve the
yield of pulmonary CT angiography? 196: 1059-1064
(160) AJR 2011; Fujikawa A, et al. Vascular enhancement and
image quality of CT venography: comparison of standard and low
kilovoltage settings. 197: 838-843
(161) J Nucl Med 2011; Glasser JE, et al. Successful and
safe implementation of a trinary interpretation and reporting
strategy for V/Q lung scintigraphy. 52: 1508-1512
(162) AJR 2011; Ridge CA, et al. Pulmonary CT angiography
protocol adapted to the hemodynamic effects of pregnancy. 197:
1058-1063
(163) AJR 2012; Nakazono T, et al. HIV-related cardiac
complications: CT and MRI findings. 198: 364-369
(164) Radiology 2012; Leung AN, et al. American Thoracic
Society documents: an official American Thoracic Society/Society
of Thoracic Radiology clinical practice guideline- evaluaiton of
suspected pulmonary embolism in pregnancy. 262: 635-646
(165) J Nucl Cardiol 2012; Einstein AJ, et al. Effect of
bismuth breast shielding on radiation dose and image quality in
coronary CT angiography. 19: 100-108
(166) AJR 2012; Wang PI, et al. Imaging of pregnant and
lactating patients: part 2, evidence-based review and
recommendations. 198: 785-792
(167) Radiology 2012; Kalb B, et al. MR imaging of pulmonary
embolism: diagnostic accuracy of contrast-enhanced 3D MR
pulmonary angiography, comtrast-enhanced low-flip angle 3D GRE,
and nonenhanced free-induction FISP sequences. 263: 271-278
(168) AJR 2012; Araoz PA, et al. Panel discussion: pulmonary
embolism imaging and outcomes. 198: 1313-1319
(169) AJR 2012; Woo JKH, et al. Risk-benefit analysis of
pulmonary CT angiography in patients with suspected pulmonary
embolus. 198: 1332-1339
(170) AJR 2012; Sheh SH, et al. Pulmonary embolism diagnosis
and mortality wit pulmonary CT angiography vrsus
ventilation-perfusion scintigraphy: evidence of overdiagnosis
with CT? 198: 1340-1345
(171) AJR 2012; Lu MT, et al. Axial and reformatted
four-chamber right ventricle-to-left ventricle diameter ratios
on pulmonary CT angiography as predictors of death after acute
pulmonary embolism. 198: 1353-1360
(172) AJR 2012; Fanous R, et al. Image quality and radiation
dose pof pulmonary CT angiography performed using 100 and 120
kVp. 199: 990-996
(173) AJR 2012; Wu CC, et al. Pulmonary 64-MDCT angiography
with 30mL of IVcontrast material: vascular enhancement and image
quality. 199: 1247-1251
(174) AJR 2013; Colletti PM, et al. To shield or not to shield:
application of bismuth breast shields. 200: 503-507
(175) AJR 2013; Mayo J, Thakur Y. Pulmonary CT angiography as
first-line imaging for PE: image quality and radiation
considerations. 522-528
(176) AJR 2013; Kim YK, et al. Reduced radiation exposure of
the female breast during low-dose chest CT using organ-based
tube current modulation and a bismuth breast shield: comparison
of image quality and radiation dose. 200: 537-544
(177) AJR 2013; Aghayev A, et al. The rate of resolution of
clot burden measured by pulmonary CT angiography in patients
with acute pulmonary embolism. 200: 791-797
(178) AJR 2014; Browne AM, et al. Evaluation of imaging quality
of pulmonary 64-MDCT angiography in pregnancy and puerperium.
202: 60-64
(179) AJR 2014; Kligerman SJ, et al. Missed pulmonary emboli on
CT angiography: assessment with pulmonary embolism-computer
aided detection. 202: 65-73
(180) JAMA 2014; Righini M, et al. Age-adjusted D-dimer cutoff
levels to rule out pulmonary embolism. The ADJUST-PE study.
1117-1124
(181) J Nucl Med 2014; Perisinakis K, et al. Perfusion
scintigraphy versus 256-slice CT angiography in pregnant
patients suspected of pulmonary embolism: comparison of
radiation risks. 55: 1273-1280
(182) Radiology 2014; Zhang LJ, et al. Pulmonary embolism and
renal vein thrombosis in patients with nephrotic syndrome:
prospective evaluation of prevalence and risk factors with CT.
273: 897-906
(183) AJR 2015; Thacker PG, Lee EY. Pulmonary embolism in
children. 204: 1278-1288
(184) AJR 2015; Hutchinson BD, et al. Overdiagnosis of
pulmonary embolism by pulmonary CT angiography. 205: 271-277
(185) AJR 2016; Geeting GK, et al. Mandatory assignment of
modified Wells score before CT angiography for pulmonary
embolism fails to improve utilization or percentage of positive
cases. 207: 442-449
(186) Ann Intern Med 2006; Le Gal G, et-al. Prediction of
pulmonary embolism in the emergency department: the revised
Geneva score. 144: 165-71
(187) Radiology 2017; Yan Z, et al. Yield of CT pulmonary
angiography in the emergency department when providers override
evidence-based clinical decision support. 282: 717-725
(188) AJR 2017; Chiu V, O'Connell C. Management of incidental
pulmonary embolism. 208: 485-488
(189) AJR 2017; Unal E, et al. Nonthrombotic pulmonary artery
embolism: imaging findings and review of the literature. 208:
505-516
(190) Radiology 2017; Sista AK, et al. Stratificaiton, imaging,
and management of acute massive and submassive pulmonary
embolism. 284: 5-24
(191) AJR 2020; Al-Hakim R, et al. Evaluation and management of
intermediate and high-risk pulmonary embolism. 214: 671-678
(192) AJR 2020; Ahuja J, et al. In situ pulmonary artery
thrombosis: unrecognized complication of radiation therapy. 215:
1329-1334