Lymphoscintigraphy
Chemistry and Pharmacology
Tc-Antimony trisulfide colloid was previously the agent of choice for nuclear lymphoscintigraphy, but this agent is no longer commercially available. Other radiocolloids are therefore used and include Tc-sulfur micro- or nanocolloid (which is Tc-Sulfur colloid filtered through a millipore filter to eliminate all particles larger than 0.22 microns in diameter) or Tc-human serum albumin (HSA). The permeability of lymphatics to colloidal particles is maximal for particles less than 50 nm. Larger particles remain mostly at the injection site.
An inherent limitation of lymphoscintigraphy is its poor visualization of the deep lymphatic system. Web space injections highlight the superficial system only. Deep lymphatic channels originating posterior to the malleoli and coursing to the popliteal nodes and then along the superficial femoral vein are usually not identified.
Micropore filtered Tc-SC in susupended in 0.1 ml of saline and 1
mCi (37 MBq) is injected intradermally in the web interspaces of
the extremities to be evaluated [20]. Tc-sulfur colloid requires
an acidic pH to remain stable and this can cause the patient to
experience burning at the injection site [23]. The area of
injection is massaged for 2 minutes immediately after injection
[20]. A resolution collimator is used and images are acquired
sequentially over the extremities. A transmission scan should also
be recorded to permit anatomic localization of the visualized
areas [23]. In patients with normal lymphatics, a predictable
sequence of tracer activity should be identified. In the lower
extremities, activity normally appears in the lymphatic bundle
along the anteromedial aspect of the leg. Several channels may be
identified in the calf, but only one (or two) vessel(s) is seen in
the thigh [20]. Bilateral visualization of the inguinal lymphatics
should be seen within one hour [20], but activity can appear
within the pelvic nodes within 10 minutes due to the small size of
the particles. Typically one to three popliteal nodes and two to
ten inguinal nodes are visualized [20]. Muscular exercise
(walking) is used to enhance lymphatic flow. Abnormal findings
include interruption of lymphatic flow, collateral lymph vessels,
dermal backflow, delayed flow, delayed asymmetric visualization or
non-visualization of lymph nodes, a reduced number of lymph nodes,
dilated lymphatics, or no visualization of lymphatic channels
[20].
Tc-tilmanocept (Lymphoseek) has also been approved for
lymphoscintigraphy [49]. The agent is a receptor targeted small
synthetic molecule (diameter 7.1 nm) that accumulates in lymphatic
tissue by binding mannose receptors (CD206) expressed on
reticuloendothelial cells in lymph nodes [50]. The agents small
size allows more rapid clearance from the injection site and its
specific binding to lymphatic tissue allows for sustained SLN
uptake in first echelon nodes [50]. The dose is 0.5 mCi.
Lymphedema:
Lymphedema results from the progressive accumulation of protein rich fluid in the interstitial spaces secondary to an anatomic or functional obstruction of the lymphatic system. Patients with lymphedema experience extremity swelling, decreased mobility, and secondary infections [20]. There is both a superficial and deep lymphatic system which drain at very different rates. The superficial system is the primary route for lymphatic drainage [23]. Deep lymphatic transport is less than 10% of the superficial transport system [23].
Primary lymphedema is the result of congenital aplastic or hypoplastic lymphatics. In lymphedema distichiasis syndrome there are mutations in the FOXC2 gene that leads to lymph reflux due to lymphatic valve failure [39]. A familial form of primary lymphedema is Noone-Milroy's disease in which there is failure of initial lymphatic uptake as a result of mutations in the gene for vascular endothelial growth factor receptor 3 [39]. Noone-Milroy's disease is a rare disorder and non-familial primary lymphedema is much more common. Primary lymphedema is classified by the age of onset- congenital, or praecox (prior to) and tarda (over the age of 35, respectively). The onset is usually spontaneous without history of trauma, surgery, or radiation [20]. The disorder is slowly progressive, tends to affect females (4:1 predominance) with a peak incidence at puberty, and a lower limb is involved in the majority of cases.
In patients with congenital lymphatic aplasia, scintigraphy typically demonstrates little to no removal of tracer from the injection site, no lymphatic channels, and no regional nodal activity. A decreased number and or caliber of lymphatic vessels is seen in patients with hypoplasia.
Secondary lymphedema occurs when there has been some type of
insult to the normal lymphatic channels (surgery, trauma,
neoplasm, or inflammation). The disorder can present months to
years after the initial injury [20]. Worldwide, the most common
cause is filariasis due to Wuchereria bancrofti infection.
Post-surgical lymphedema is also common following axillary node
dissection (5-30%) and pelvic cancer surgery (10-49%) [23].
Microangiolymphatic surgery is usually more successful in
secondary lymphedema than in primary lymphedema. Scintigraphy
typically demonstrates delayed transport of tracer from the
injection site with dermal back flow, large collateral channels,
and fewer visualized lymph nodes. Cross-over of activity from the
contra-lateral normal side to pelvic nodes on the affected may
also be seen and care should be taken to ensure that this finding
is not misinterpreted as normal.
Sentinel Node Detection:
Clinical: The prevalence of malignant melanoma has rapidly increased and the lifetime risk for melanoma in the United States will exceed 1% in the near future [4]. Presently, data from 2003-2205 indicate a lifetime risk of about 1.8% [21]. Melanoma can be cured by surgery alone when the disease is localized [8]. However, up to 20% of patients that present with melanoma and no clinical evidence of metastases harbor occult lymph node metastases. Patients at greatest risk for lymph node metastases are those with a lesion depth of more than 1 mm Breslow thickness. The incidence of sentinel lymph node metastasis is approximately 5% for melanomas between 0.76 and 1.5 mm in thickness, but increases to almost 20% for melanomas between 1.5 and 4 mm [8]. For patients with clinical stage I-II melanoma, the status of the sentinel lymph node is the single most important factor for determining overall survival (independent of primary tumor thickness) [7,43]. The presence of regional lymph node metastases is associated with a 5 year survival of 73% compared to 97% with negative SLN biospy [3,6,8]. Prophylactic elective lymph node dissection can result in a 50% 5 year survival rate when micrometastases are found (as compared to a 25% survival rate when patients subsequently present with enlarged lymph nodes) [3]. Unfortunately, morbidity from lymph node dissection is high and the wrong field can be dissected in up to 30% of patients [6].
In-transit nodes represent embryonic rests of lymphatic tissue that can be found anywhere along the pathway of a lymphatic channel and these rests may form a lymph node that lies outside the expected nodal basin [21]. Because the incidence of metastatic in-transit or aberrant nodes in melanoma can be between 14-22%, lymphoscintigraphy with lymph node mapping can be used to better delineate the lymphatic drainage from the primary lesion [33]. The procedure is based on the hypothesis that melanoma metastasizes to regional lymph nodes via a defined connection of dermal lymphatics which can be followed to the first or "sentinel" lymph nodes in the regional basin. The absence of tumor in the sentinal node is felt to be strongly indicative of the absence of metastases to other nodes in the regional basin (skip metastases to second or third tier lymph nodes with sparing of the sentinel node occurs in less than 2% of patients [8]). Thus, the patient could be spared extensive node dissection surgery if the sentinel nodes are negative. If the sentinel nodes are positive for tumor, then the patient would undergo a complete lymph node dissection [2]. The success rate of lymphoscintigraphy for sentinel node identification in approximately 98% [8]. SLN biopsy is associated with a lower rate of post-operative complications (about 10% of patients compared to 40% after elective node dissection) [6]. Previous surgery to a node field can cause unusual drainage pathways, but lymphoscinitigraphy can still accurately stage the draining lymph nodes [6].
Procedure: Filtered Tc-sulfur colloid (1 mCi) is
injected intradermally in 4 to 6 equal doses of 0.1 to 0.2 mL
about the lesion or surgical site (about 0.5 to 1 cm from the
lesion) [4,8,21] (a minimum of three injections is recommended
[7]). Small injected volumes (no larger than 0.2 mL) should be
used to decrease the risk of collapsing local lymphatics [8,21].
Only about 5-8% of the injected dose will migrate from the
injection site [5]. Imaging is started immediately after injection
using a large field of view detector with a parallel hole
collimator, a 10% window centered on the 140-keV technetium energy
peak, and a 128 x 128 matrix [4]. Dynamic images are obtained at
20-30 seconds per frame for 30 to 45 minutes to demonstrate
lymphatic flow and regional drainage. Other authors recommend
acquiring dynamic images at 10-15 seconds pre frame for 10-15
minutes [21]. Following the dynamic phase, static images should be
obtained every 5 minutes for 45-60 minutes (it is unlikely for a
sentinel node to first appear beyond 45-60 minutes after
injection) [21]. Lateral or oblique static images are also
acquired for 300 seconds using a 256 x 256 matrix to better depict
the nodal findings [8]. An outline of the patients body should be
obtained using a radioactive flood source to aid in image
orientation [4]. Delayed static images at 1, 2, and 4 hours can be
performed with static 5-10 minute images to evaluate all regions
that could possibly drain the primary melanoma (a transmission
source on the delayed images helps to highlight the body outline)
[5]. Activity from the injection site may occasionally mask the
regional nodes if they are located very near to each other.
Shielding may be required.
SPECT imaging on hybrid SPECT/CT systems can aid in
identification of nodes that are not seen on planar images,
obscured by injection site activity, and for deeply located and
in-transit nodes [33,51]. SPECT /CT can also aid in confirming the
exact anatomic location of a sentinel node when compared to planar
imaging [9,10,43]. SPECT/CT imaging has been shown to be
associated with excision of more sentinel lymph nodes per patient
compared to planar imaging and the surgical approach can be
changed in up to 22% of patients [43]. SPECT/CT is particularly
useful for the localization of sentinel nodes in the head and neck
region and in pelvic malignancies and is superior to planar
imaging [43,51]. The addition of SPECT/CT as part of the
lymphoscintgraphy evaluation of patients with melanoma has been
shown to be associated with prolonged disease free survival (93.9%
versus 79.2%) [43]. The false negative rate is also lower with
SPECT/CT (6.8% versus 23.8%) [43].
The sentinal node is usually evident as the primary lymphatic channel will drain to this node. The sentinel node detection rate can be as high as 99.3% [7]. More than one primary lymphatic channel may be identified and therefore, patients can have more than one sentinal node. Another benefit of lymphoscinitgraphy is that unanticipated patterns of lymphatic drainage can be identified and aid in directing surgical node dissection. Between 7.5-15% of melanoma patients can have dual drainage patterns [2]. For the procedure, the surgeon utilizes a hand-held gamma probe to identify radioactive nodes. Blue dye is also generally injected intradermally just prior to the procedure [3]. The count rate is higher in sentinal nodes and the first node in the line is usually the most radioactive [1]. Generally a count rate at least 3 times that of background [3] or a node with a count rate of at least 10-20% of the count rate in the hottest removed node is considered positive [11]. Blue staining of the node helps to confirm that the node is the sentinel node [3]. After a positive sentinel node is identified, complete lymphadenectomy of the lymphatic basin should be performed [8,11]. The disease free survival rate increases from 73% to 78.5% when complete lymph node dissection is performed immediately after a positive SLN biopsy, than when nodal metastases become clinically evident [11]. Lymphadenectomy alone has improved the 5 year survival rate from 27% to 48% [8].
Outcome: Lymphatic mapping with sentinel node dissection
has been demonstrated to be equivalent [2] and possibly superior
[8] to elective lymph node dissection. The procedure can provide
prognostic information based upon the presence or absence of
regional lymph node metastases [3,8]. Although the procedure
reduces the morbidity associated with elective lymph node
dissection, lymphoscintigraphy and sentinel node dissection has
not been show to result in overall disease-free survival [2]. The
rate of regional lymph node recurrences is also similar between
the two procedures [2]. Additionally, there is controversy about
the procedure due to the lack of elective adjuvant therapy once
sentinel node metastases have been identified [8].
The exam has been shown to be very reproducible (96% of cases), but variations in lymphatic drainage can be seen between two exams, particularly for tumors located in the head and neck region or on the trunk [42]. The reported false negative rate of SLN biopsy ranges from 10-15% (i.e.: patients in whom metastatic disease appears during follow-up in a basin classified as disease free on the basis of a negative SLN biopsy) [11]. Macrophage function, and hence retention of radiocolloid in the node, is lost as the amount of metastatic involvement in the node increases and this nodal replacement by tumor can result in false-negative exams [21].
REFERENCES:
(1) J Nucl Med 1997; Kapteijn BA, et al. Validation of gamma probe
detection of the sentinal node in melanoma. 38: 362-366
(2) Ann Surg Oncol 1999; Essner R, et al. Efficacy of lymphatic mapping, sentinel node lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. 6: 442-449
(3) Cancer 2001; Statius Muller MG, et al. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with stage I or II cutaneous melanoma. 91: 2401-08
(4) Radiographics 2002; Lymphoscintigraphy in cutaneous melanoma: a total body atlas of sentinel node mapping. 22: 491-502
(5) J Nucl Med 2003; Uren RF, et al. Patterns of lymphatic drainage from the skin in patients with melanoma. 44: 570-582
(6) J Nucl Med 2006; Yren RF. Sentinel node biopsy in melanoma. 47: 191-195
(7) J Nucl Med 2006; Rossi CR, et al. The impact of lymphoscintigraphy technique on the outcome of sentinel node biopsy in 1,313 patients with cutaneous melanoma: an Italian mutlicentric study (SOLISM-IMI). 47: 234-241
(8) J Nucl Med 2002; Mariani G, et al. Radioguided sentinel lymph node biopsy in malignant cutaneous melanoma. 43: 811-827
(9) J Nucl Med 2007; van der Ploeg IMC, et al. The additional value of SPECT/CT in lymphatic mapping in breast cancer and melanoma. 48: 1756-1760
(10) J Nucl Med 2008; Buck AK, et al. SPECT/CT. 49: 1305-1319
(11) J Nucl Med 2008; Manca G, et al. Optimal detection of senitnel lymph node metastases by intraoperative radioactive threshold and molecular analysis in patients with melanoma. 49: 1769-1775
Background:
Axillary node status is required to properly stage breast cancer [3]. The presence or absence of axillary node metastases carries important prognostic information [3] and guides adjuvant therapy [18]. There is a 20-30% likelihood for the presence of axillary nodal metastases in very early breast cancers (T1a-b, tumor size less than or equal to 1 cm), and this rises to 30-40% for T1c lesions (size 1-2 cm) [18]. The rate reaches 45% for small T2 tumors (2.1-3cm) and 55-70% for larger tumors [38]. Clinical exam has a poor predictive value for the determination of nodal involvement and therefore histologic examination of the axillary nodes is important for the identification of metastatic disease [18]. Conventional axillary lymph node dissection involves extensive dissection of most of level I, II, and III nodes [28]. Unfortunately, conventional axillary node dissection surgery suffers from sampling error and can be complicated by significant patient morbidity (particularly lymphedema and sensory-motor disturbances in the ipsilateral arm) [3,18].
The "sentinel node" is the first lymph node encountered by lymphatic vessels draining a tumor [18]. The absence of tumor in the sentinal node (SLN) is felt to be strongly indicative of the absence of metastatic disease to other nodes in the regional basin. Thus, patients could be spared extensive node dissection surgery if the sentinel node could be identified, examined, and found to be free of tumor. Further axillary dissection could be performed only in patients with positive findings for malignancy in the sentinal node or in whom the sentinel node could not be localized. Sentinel node biopsy would represent a significant advantage as a minimally invasive procedure, considering that, after surgery, about 70% of patients with clinically N0 disease are found to be free from metastatic disease [18]. On a size basis, a metastasis in a sentinel node is designated a macrometastasis when larger than 2mm, a micrometastasis (pN1mi) when 0.2mm to 2mm, and isolated tumor cell clusters (pN1i+) when 0.2 mm or smaller and having no more than 200 tumor cells in a single section [38]. The relationship of micrometastases or isolated tumor cell clusters to disease free survival is controversial with some authors suggesting no difference from node negative patients, while other indicate the finding is clinically relavant [38]. The tumor deposits detected solely by anticytokeratin immunostaining could be benign epithelial cells transported to the sentinel lymph node following breast biopsy [38].
Lymphoscintigraphy has been used in the detection of the sentinal
node in patients with breast cancer. The axillary sentinal node
can be properly identified in 90 to 100% of patients with use of
an intraoperative gamma detecting probe and blue dye [2,10,11].
The technical success rate for the exam has been reported to be
between 66% to 100%. The exam has a reported sensitivity of
83-100% (rough average of 95-97%), specificity of 100%, positive
predictive value of 100%, negative predictive value of 92-100%,
and an accuracy of 95-100% [4,18]. The exam has been shown to be
highly reproducible [25], accurate [30], and associated with less
morbidity compared to axillary node dissection [30]. However,
recent studies have suggested the false negative rate to vary
considerably between 5.5-16.7% (average 9.2%) [38]. The number of
resected nodes will affect the false negative rate- with higher
rates associated with resection of only one or two lymph nodes
[38]. A higher tumor grade is also associated with a higher false
negative rate [38]. Careful palpation by the surgeon is also
required to identify any large, hard nodes that might not be
radioactive [38]. There is no evidence that SLN biopsy is
associated with an increase in local recurrence in the regional
node field [32].
Residual axillary node disease is found in 50-60% of patients
following neoadjuvant therapy for clinical node positive disease
[44]. Among women that have received preoperative chemotherapy for
biopsy proven node positive breast cancer, lymphoscintigraphy can
have a false negative rate up to 12.6% [44]. This may be seen as
after chemotherapy the axilla often has more fibrosis that can
make evaluation of lymphatic drainage and surgical dissection more
challenging [44]. Factors associated with a higher rate of false
negative SNL in this setting include removal of fewer than 2 SLN
and lack of dual-agent mapping [44].
The rationale for sentinel lymph node mapping using scintigraphy is based on the fact that under normal circumstances the breast (the mammary gland and the overlying skin) can be considered a single biological unit with a common centrifugal lymphatic pathway [29]. Lymph flows from the superficial to the deep layers and then towards regional lymph nodes through lymph channels that originate in the interlobular spaces and along lactifierous ducts [29]. The lymphatic vessels of the breast tend to accompany the routes of blood supply- mainly the axillary and internal mammary vessels [18]. Most of the lymph drains to the axillary lymph nodes [29]. About 3% drains to the internal mammary chain nodes (usually from the medial portion of the breast), and a very small percentage drains to the posterior intercostal nodes [18,29]. Part of the lower portion of the breast can drain to the lymphatic system of the upper abdominal wall, and part of the upper portion can drain to the apical axillary nodes and deep cervical nodes [18]. Thus, the pattern of lymphatic drainage can be variable and it is impossible to predict the route of drainage of a tumor based upon its location within the breast [18]. Although the presence of "skip" metastases (axillary node metastases to second-tier nodes or elsewhere with no evidence of metastases at level I) has been reported to be between 4-12% [4,18]. These "skip" lesions likely represent sentinel nodes outside level I and such nodes can be readily identified by lymphoscintigraphy [4].
|
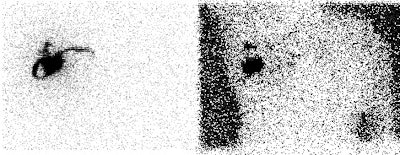
Internal mammary node drainage: The patient underwent lymphoscintigraphy as part of a sentinel node dissection procedure. An intradermal injection was performed in the right breast at the 2 o'clock position over the patients breast cancer. An anterior and transmission image are shown below. Drainage to both the right axillary and right internal mammary nodes (black arrow) can be seen. Although the internal mammary nodes were not dissected at surgery, it is important to note this activity if post operative adjuvant radiation therapy is planned. |
|
|
Lymphoscintigraphy can provide information regarding primary lymphatic drainage to sites other than the axilla such as the inaccessible internal mammary (IM) chain (which occurs in 6-20.4% of cases [3,10,19,26,38]). Although internal mammary (IM) node drainage is more commonly seen with tumors located in the inner quadrants [19], it is important to note that up to 46% of tumors with drainage to IM nodes can be located within the outer part of the breast [10]. In patients that demonstrate internal mammary nodal uptake at lymphoscintigraphy, likelihood for metastases is higher if axillary lymph node metastases are found at biopsy (41% versus 5-8% if axillary nodes are negative) [38]. Some authors have suggested that because of the high risk of occult internal mammary involvement in cases of positive axillary sentinel node and internal mammary drainage at lymphoscintigraphy, that these patients be offered internal mammary radiotherapy without biopsy of the IM nodes [38].
Drainage to internal mammary sentinel nodes is more likely to be identified when peritumoral tracers injections are performed [27,38]. IM nodal involvement is considered N2 disease and upstages patients to a minimum of clinical stage III disease [53]. Even in the absence of biopsy, the presence of IM nodal drainage at scintigraphy portends a worse distant disease-free survival [53]. Patients with IM and axillary node metastases have a significantly worse prognosis than patients with isolated axillary node disease [19]. Despite this fact, pursuit of sentinel nodes outside the axilla is not performed at all institutions. This is because the treatment of IM nodal disease remains controversial [24]. Surgical resection of IM nodes may not reduce local-regional recurrence or improve survival [24], although aggressive radiation therapy may provide some benefit [19]. Lymphoscintigraphy does permit evaluation of IM nodes and may enable more accurate staging and spare patients unnecessary axillary node dissections [17].
Indications/Contraindications:
Patients who are being considered for axillary node dissection
and who do not have clinically involved nodes by exam or high
tumor grade (T3 or T4, tumor larger than 4 cm) are candidates for
lymphoscinitgraphy. Patients with nodes that are felt to be
involved by tumor on clinical exam are not candidates for
lymphscinitgraphy as lymphatic flow to a node that is replaced by
tumor may be obstructed and the node may not be identified (ie:
incorrect localization of the sentinel node) [12,14,18,35].
Patients with multifocal or multicentric cancers are also
generally not candidates for lymphoscintigraphy as the procedure
may not reflect all possible sentinel nodes from different lesions
[18,22]. However, at least one study has shown very good
sensitivity and negative predictive value in this subgroup of
patients [22]. Surgical excisional biopsy can disrupt lymphatic
pathways and may affect exam results [4,38], although other
authors have found no difference in sentinel node localization
following prior surgical biopsy [10,11,15]. Patients who have
undergone prior surgical procedures in the axilla can have altered
patterns of regional lymphatic drainage and should be evaluated on
a case-by-case basis [18].
Patients that have received pre-operative adjuvant chemotherapy
may not be good candidates for lymphoscintigraphy as there can
considerable variability in sentinel node detection rates (63-100%
[55,56]) and between 12.6% and 33% of patients can have a
false-negative result - possibly related to lymphatic
changes, fibrosis, and patchy killing of metastatic foci
[23,38,50]. In the ACOSOG 1071 and SENTINA trials, when at least 3
sentinel lymph nodes were obtained, the false negative rates were
12.6% and 14.2% respectively (which is higher than the usually
accepted FNR of 10%) [56]. To reduce the FNR, retrieval of more
than two SLN and using dual-mapping techniques with both blue dye
and scinitgraphy are recommended [56]. In the ACOSOG trial, adding
the presence of a normal a axillary US to SLN biopsy reduced the
FNR to 9.8% [56]. If an abnormal LN is detected at post NAC
axillary US and the node is biopsied and found to be positive, a
clip should be placed to allow identification and removal of the
node at surgery [56].
The National Comprehensive Cancer Network recommends that lymphoscintigraphy be performed prior to adjuvant treatment [38].
Agent/Procedure:
Tc99m-sulfur colloid (Tc-SC) is the agent most commonly used for sentinel node localization. Unfortunately, there is no standardized technique for sentinel node localization. Wide variations exist regarding particle size, timing of injection, volume and placement of the injection, use of pre-operative imaging, and operative techniques [11]. It is important to avoid skin contamination during the injection [28]. Methods to decrease skin contamination include applying negative pressure to the syringe during needle withdrawal and immediately dabbing the injection site with a gauze pad [28].
Particle size: Radiocolloids are cleared by lymphatic drainage with a speed that is inversely proportional to the particle size [18]. A particle size between 100-200 nm is probably the best compromise between the need for efficient and fast lymphatic drainage and nodal retention of the particle [18]. Particles larger than 300 nm migrate too slowly (although they are retained for a greater period of time within the sentinel node), while particles less than 50 nm progress to second or third-tier nodes too quickly [18]. The Tc-SC particle size can be reduced to aid in lymphatic uptake of the tracer. A reduction in particle size is accomplished by filtering the agent through a 200 nm millipore filter, use of shortened boiling times during preparation or by using Tc-pertechnetate of longer ingrowth [3]. Not all centers use filtered colloid as they feel there is improved/prolonged retention of the agent in the sentinal nodes when the colloid is not filtered [4,9,11].
pH: Some centers adjust the pH of the injected agent to neutral with sodium bicarbonate [5].
Injection site: Peri-tumoral, intradermal, or intra-lesional injection can be performed. Injection into a biopsy cavity, seroma, or hematoma may result in poor or no migration [3] and is not recommended. Prior to surgical biopsy, 1% isosulfan blue dye is also commonly injected around the margins of the tumor (intraparenchymal) in order to aid in SLN localization. The combination of isotope and blue dye have a complementary effect in sentinel node localization [10-12,14,18,38]. The risk of allergic reaction is close to 1% for the blue dye (0.1% risk for severe reaction) [38].
1- Intraparenchymal/peritumoral injection: It would seem logical that a peri-tumoral injection of the tracer would most accurately reflect the lymphatic drainage of the lesion [11]. Approximately 0.5 - 1.0 mCi of the agent in a total volume of 3 to 7 ml is injected in 4 to 6 deposits around the periphery of the tumor [3,18]. If the lesion is not palpable, a single injection can be performed through a localizing wire introducing needle [5] or using ultrasound guidance. A peri-tumoral injection will generally have slower tracer clearance than an intradermal exam- especially in patients with large breasts or post-menopausal women (possibly due to reduced lympahtic flow in the aging breast parenchyma) [18]. Images are usually obtained 45 minutes to 1-2 hours after injection [28]. A gentle massage for 2-3 minutes after the injection may aid clearance [18]. Due to the several injections made with this technique, there may be a "blooming" of the tracer activity which can obscure the axilla or internal mammary node area on imaging [11]. Some authors also report a decrease in SLN identification with this technique compared to an intradermal injection [11,17], however, there is a high rate of visualization of internal mammary sentinel nodes [18] and some authors suggest that only preilesional injection provides an accurate depiction of extra-axillary drainage, especially to internal mammary nodes [36]. If visualization of internal mammary lymph nodes is deemed important, then a peritormal injection should be performed [28]. Some sentinel nodes can also be identified in the breast parenchyma, in between the pectoralis muscles, and in the supraclavicular fossa [18].
2- Intra/subdermal injection: Because of its embryologic origin in the ectoderm, the mammary gland is an organ of the skin and its lymphatic drainage mostly parallels lymph flow from the overlying skin [18]. For the dermal injection technique, a single injection of the tracer is made in the skin directly overlying the tumor [18]. Dermal injections are generally performed using a smaller dose and volume (0.1 to 0.6 mCi in 0.15 to 0.5 mL) [9,18]. The lower injected activity is associated with less "blooming" and the technique is easier to perform than peri-tumoral injections [11,14]. Tracer migration is also faster using a dermal injection with visualization of the sentinel node as early as 20-30 minutes following injection [16,18]. Additionally, this type of injection yields more radioactive counts at the axillary sentinel nodes [38]. Localization of the SLN with this technique is excellent [10,11,14] and a dermal injection may actually be superior to a peri-tumoral injection for SLN identification [11,15]. A potential drawback of dermal injections is that they may be less likely to drain toward the deep fascial lymphatic channels and have a lower detection of intramammary nodes (i.e.: non-axillary sentinel nodes) [16-18]. The incidence of metastatic involvement of internal mammary nodes in patients with T1 (up to 2 cm diameter) breast cancer has been reported to be as high as 15%, but a much lower value has also been reported (about 3%) [18]. A reasonable approach might be to inject the radiocolloid intradermally when evaluating a T1a-b tumor (less than or equal to 1 cm in diameter) located superficially in the breast, and inject peritumorally in the case of larger tumors or tumors located deep within the breast [18]. A combination of peritumoral and intradermal injections can also be performed [28].
3- A single intratumoral injection is an alternative method and is also a valid way to identify the lymphatic drainage from the lesion [6]. There are several drawbacks to an intratumoral injection. The tumor itself is intrinsically devoid of an organized lymphatic system and drainage is often very slow [18]. Even 18-24 hours after injection, a large fraction of the injected dose is retained within the tumor [18]. Scatter or shine-through associated with this retained activity can interfere with imaging and gamma-probe use during surgery [18]. Although rare, an intratumoral injection could also spread tumor cells along the needle track [18].
4- Periareolar region injection: In the breast, there is a rich subareolar lymphatic plexus (the plexus of Sappey) [29]. Injections are performed adjacent to the areola in the tumor quadrant [28]. Activity reaches the axilla at a similar or faster rate than for dermal injections [28]. However, with the use of periareolar injections, there is less "shine-though" of activity which can sometimes interfere with the exam- particularly when injections are made in the upper-outer quadrant of the breast [29]. The sentinel lymph node detection rate is about 98% [29]. As with other superficial injection techniques, detection of drainage to internal mammary lymph nodes is decreased when compared to peritumoral injections [29].
Imaging:
A large-field-of-view camera is useful to obtain the draining pattern of the entire lymphatic basin [18]. The energy setting of the camera should be centered on the 140-keV emission peak of Tc-99m, with a +/- 10% window. The use of a high-resolution collimator and an acquisition matrix of 256x256 is recommended [18]. Imaging should begin within a few minutes of injection, however, dynamic imaging is not required as it is for sentinel node localization in melanoma [16]. The patient should be placed in a supine/semirecumbent position with the ipsilateral shoulder elevated to about 45 degrees by a triangular foam wedge and the arm raised over the head. This position helps to separate injection activity from axillary node activity [7] and allows the head of the gamma camera to be placed as close as possible to the axilla [18]. In patients with large breasts, it is sometimes useful to move the breast to clear the axillary region and reduce the attenuation effect [18]. Imaging is started with an anterior projection, but can be changed to an oblique anterior view with some craniocaudal tilting as necessary to distinguish between injection and nodal activity [18]. At completion of the exam an anterior image is still useful to obtain for evaluation of activity in the internal mammary nodes [7]. Nodes located close to the injection site may sometimes be obscured by scatter radiation. Adjusting the energy window to sample only the high side of the technetium photopeak can assist in identifying these nodes (ie: use 15% energy window centered at 151 keV) [3]. Timing of the sequential spot images should be based upon the size of the colloid particles injected and the type of injection used (peritumoral vs intradermal) [18]. The mean time to visualization of the sentinel node is about 20 minutes [3], but imaging can take up to 2 hours. An outline of the patients silhouette traced with a Tc-99m point source (< 0.5 mCi) or with a Co-57 sheet source behind the patient aids in image interpretation [3,18].
|
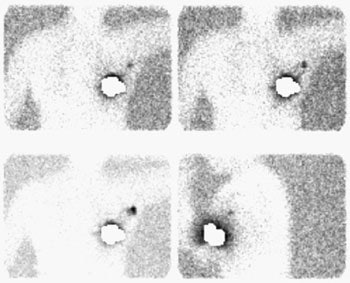
Sentinel lymph node imaging: The lymphoscintigraphy exam below was performed using a peri-tumoral tracer injection. Images were performed at 5, 10, and 15 minutes after injection in a LAO projection with the left arm behind the patient's head. A lead shield was placed over the breast. The exam demonstrated rapid localization of the primary lymphatic drainage to the axilla. Following the procedure the patient went to the operating room for gamma probe localization of the sentinel node. |
|
|
|
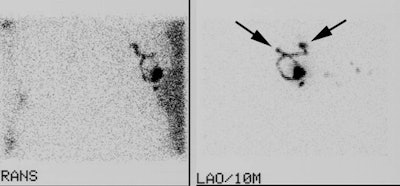
Two sentinel lymph nodes: It is important to document if there is more than one drainage track from the injection site. In the case below, LAO and transmission images demonstrate the presence of two sentinel lymph nodes (black arrows). It is very important that this finding be communicated to the surgeon. |
|
|
Once identified, the sentinel node should be further localized and marked on the skin with an indelible marker using a hand-held probe with the patient in the same position that will be used in surgery (generally the arm should be abducted to 90 degrees [18]). Marking the approximate position of the sentinel node may assist the surgeon in reducing operating time and in keep the surgical incision to a minimum [18]. If imaging is delayed, multiple nodes may be seen and localization of the sentinel node may not be possible. If a sentinel node is not identified on scintigraphic imaging, a sentinel node can still often times be localized at surgery- either with a handheld probe or with blue dye [10]. However, in patients that fail to have a sentinel node identified during imaging, there is a higher rate of failure to identify a SLN at surgery (19% of patients with non-visualized SLN on imaging had no SLN identified at surgery, as compared to only 1% of patients with visualized SLN at imaging) [10]. Patients over the age of 50-60 years are also more likely to have failure to localize the sentinel node [10,11].
A two day protocol has also been evaluated using unfiltered Tc-sulfurcolloid with results similar to the one day exam (sensitivity for sentinel node localization was 97%) [9]. This protocol permits greater flexibility for patients and operating room time is not wasted waiting for patients to arrive from lymphoscintigraphy [9]. A higher dose of Tc-sulfurcolloid is used (0.5 mCi or 18.5 MBq) to allow for radioactive decay of the agent.
At surgery, a hand-held gamma probe is used to localize the sentinel node. The count rate in the sentinel node should be at least 3 to 5 times axillary background [5,9,15]. Detection of the sentinel node can be affected by the type of probe being used- with scintillation probes having the best sensitivity [31]. Often, surgeons will also pre-operatively (5 to 10 minutes before axillary dissection) inject 1% isosulfan blue dye around the tumor site to aid in the visual identification of the sentinel node and lymphatic drainage. Blue due localization is complementary to lymphoscintigraphy and can identify a small percentage of sentinel nodes (3.5-11%) not found by isotope localization [9,15]. Following removal of the sentinel node the operative site should be re-examined to ensure that the area of radioactivity has been removed and that a second node is not also active [18]. More than one sentinel node is found in a relevant number of patients (generally 1.5-1.8 per patient) [18]. All radioactive nodes should be excised until no areas of increased counts are found [18]. Gamma probe localization without lymphscinitgraphy imaging may be adequate for sentinal node localization [4,10]. However, images often help the surgeon by demonstrating the location and number of sentinel nodes, as well as unusual secondary routes of lymphatic drainage [24,26]. Additionally, visualization of sentinel lymph nodes at lymphoscintigraphy is predictive of a technically successful sentinel node biopsy procedure [24]. It has been suggested that a complete axillary lymph node dissection is associated with an improved outcome in patients with sentinel lymph node macrometastases (>2mm) [38].
Failure to identify the sentinel node is an indication for standard axillary dissection [3] as lack of identification of this node does not exclude the presence of axillary metastases [10]. In fact, failure to visualize a sentinel node suggests a higher likelihood for axillary involvement [38]. Meticulous intraoperative frozen section histopathologic analysis is performed on the node with the highest count rate, as well as on any additional lymph nodes with counting rates at least 20% of the count rate in the hottest node [18].
|
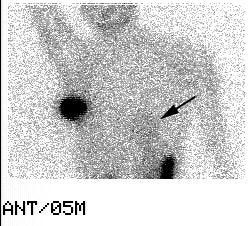
Sentinel lymph node imaging: The patient underwent lymphoscintigraphy as part of a sentinel node dissection procedure. An intradermal injection was performed in the right breast. Five minute post injection images revealed unexplained tracer activity below the diaphragm. On questioning, the patient had underwent a pre-operative myocardial perfusion examination the previous day using Tc-myoview. Note that faint cardiac activity can also be seen (black arrow). Luckily, the sentinel node procedure was not effected by this activity. |
|
|
Dosimetry:
It is important to establish procedures for handling/processing specimens, monitoring personnel, and monitoring work areas for radiation for lymphoscintigraphic localizations [3]. The dose to the breast tissue is estimated to be approximately 0.03-0.8 mSv/MBq (0.6 - 14.8 mSv for a 0.5 mCi injection) [52]. The estimate can vary by an order of magnitude depending on the volume of distribution and washout rate [3]. For peritumoral injections, resection of the breast tumor during surgery will help to decrease the radiation burden [3]. The estimated dose to a patient from a transmission scan is about 0.003 mSv [52]. The amount of radioactivity actually retained in the sentinel node is about 1% of the injected dose (subdermal injection) [18]. The surgeon receives a hand dose of about 9.4 +/- 3.6 mrem per case [3]. The cumulative dose to personnel involved in the procedure (surgeons, nurses, pathologists) for 100 operations is at most about 1% (mean absorbed dose) or about 10% (mean effective dose) of the annual dose limits for the general population [18]. The radioactivity counted in the oeprating room material possibly contaminated during surgery is generally minimal and special handling procedures are not required [18]. The simple precaution of letting radioactivity decay for some hours is sufficient for tissue specimens in the pathology department [18]. In pregnant patients, sentinel node procedures produce negligible fetal exposure of 0.014 mGy or less [34].
Conclusion:
Sentinel node localization is a team effort that requires close
coordination between the surgical team, radiology and nuclear
medicine. The surgeon and nuclear medicine physician should have a
clear understanding regarding the meaning of skin marks, patient
position, and sources of artifacts [3]. The use of both a blue dye
and radioscinitgraphy will give the benefit of visual cues and
count-rate data for the surgeon [3,4]. A learning curve exists,
and success improves with experience [4]. Each institution must
set up a program with strict technical guidelines to confirm the
accuracy of the examination [12]. The American Society of
Breast Surgeons recommends that surgeons anticipating the use of
sentinel node biopsy as their sole means of staging the axilla
should first perform 30 validation cases with accurate
identification of the sentinel node 85% of the time and a
false-negative rate less than or equal to 5% [13].
Ultrasound of axillary lymph nodes:
Certain morphologic features can aid in the identification of
pathologic lymph nodes in the axilla [45]. Findings that suggest
tumor involvement include: a focal cortical bulge/lobulation or
thickening (this is the earliest morphologic change in the
presence of metastases, but the finding has a low positive
predictive value because it is non-specific), diffuse cortical
thickening greater than 3 mm (relatively non-specific finding that
can also be seen in reactive lymph nodes), rounded hypoechoic node
(highly specific in the setting of invasive cancer), complete or
partial effacement of the fatty hilum (highly specific in the
setting of invasive cancer), non-hilar cortical blood flow on
Doppler imaging (non-specific unless combined with another
finding), coplete or partial replacement of the node with an
ill-defined or irregular mass, and microcalcifications in the node
[45,47].
Sentinel Node Detection
It
is recommended that nodal sampling be performed for patients
with a nomogram-assessed risk of over 5% for LN metastases
[54]. Extended pelvic lymph node dissection is the gold
standard for LN staging in prostate cancer, but is associated
with a high morbidity, with a 20% incidence of complications
such as infected lymphoceles and lymphedema [54]. Sentinel
node lymphadenectomy has been shown to be accurate for lymph
node staging for prostate cancer [37]. The exam is usually
performed on intermediate-prognosis patients (clinical stage
> T2b, PSA > 10 ng/mL, or Gleason score >6) who elect
radiotherapy treatment because staging can have therapeutic
consequences in these patients [37]. Since unexpected
locations of SN's outside the field of an extended pelvic
lymphadenectomy are not rare, it is crucial that SNs are
accurately identified preoperatively, as well as
intraoperatively [40]. The agent is usually injected
peritumorally guided by transrectal US [37,40,46]. The
location of tracer injection can affect the number of sentinel
lymph nodes identified [40]. More sentinel LNs are visualized
when the tracer is injected in the peripheral zone compared to
the central zone, and mid gland injections also result in a
greater number of sentinel nodes compared to injections does
near the base or apex of the prostate [40].
Indocyanine
green
(ICG)-Tc-99m-albumin
nanocolloid
is an agent that is being used in Europe for prostate
lymphoscintigraphy [40]. The agent combines a flurescent dye
(indocyanine green) with the radiotracer to permit both
optical and gamma probe identification of sentinel lymph nodes
[41].
Planar
imaging
(anterior
and
lateral 5 minute images) is performed 15 minutes and 2 hours
following tracer injection [37,40]. The use of SPECT/CT can
identify additional sentinel lymph nodes (in up to 63% of
patients- particularly nodes located near the injection area)
and aid in lymph node localization [37,40]. SPECT/CT can
identify sentinel nodes outside the planned extended pelvic
lymphadenectomy area in up to 35% of patients and in over half
of these cases, these nodes would have been missed on planar
imaging [37]. In another study, SLN were located outside the
range of limited LN dissection (obturator fossa) in 74% of
patients which highlights the need for extended lymph node
dissection [46].
The
reported false negative rate is up to 8.5% (i.e.- negative
sentinel LN with evidence of metastases to non-sentinel nodes)
[46]. Also- in patients with unilaterally identified SLN,
patients should undergo complete contralateral lymphadenectomy
because ipsilateral SLN status does not represent the nodal
status in the contralateral pelvis [46].
Sentinel
lymph node biopsy can identified significantly smaller lymph
node metastases (median diameter 2 mm) compared to 68Ga-PSMA
PET (median diameter 5.5 mm) [54]. However, in one study using
extended LN dissection as the gold standard, combining
sentinel lymph node biopsy with 68Ga-PSMA
resulted in an overall sensitivity of 100% and an accuracy of 94%
for LN staging in intermediate and high risk newly diagnosed
prostate cancer patients (PMSA PET alone detected pN1 disease in
74% of patients; 3 of 53 patients had false positive findings on
PMSA PET imaging) [54].
Cervical
cancer
Lymphoscintigraphy
can also be performed in cervical cancer staging [48].
SPECT/CT has been shown to be superior to planar imaging for
sentinel node detection and also provides superior anatomic
localization [48]. The reported false negative rate is 1.3%
[48].
Head
and neck cancer:
Oral
cavity, oropharyngeal, and supraglottic squamous cell
carcinomas can reveal occult metastases in 15-60% of cases
[50]. Patients that may be considered for lymphoscintigraphy
are those that present with stage T1 or T2 and are clinically
negative for LN metastases by exam and imaging studies [50].
The agent is injected in small volumes up to 0.2 mL containing
0.5 mCi per injection in the healthy mucosa surrounding the
malignant lesion [50].
REFERENCES:
(1) J Nucl Med 1997; Pijpers R, et al.
Impact of lymphoscintigraphy on sentinal node identification with
technetium-99m-colloidal albumin in breast cancer. 38: 366-68
(2) J Nucl Med 1998; De Cicco C, et al. Lymphoscintigraphy and radioguided biopsy of the sentinel axillary node in breast cancer. 39: 2080-2084
(3) Semin Nucl Med 1999; Glass EC, et al. Sentinel node localization in breast cancer. 29 (1): 57-68
(4) Radiology 1999; Liberman L, et al. Sentinel lymph node biopsy after percutaneous diagnosis of nonpalpable breast cancer. 211: 835-844
(5) Radiology 1999; Eary JF, et al. Sentinel lymph node mapping for breast cancer: Analysis in a diverse patient group. 213: 526-529
(6) J Nucl Med 2000; Valdes-Olmos RA, et al. Evaluation of mammary lymphoscintigraphy by a single intratumoral injection for sentinel node identification. 41: 1500-1506
(7) J Nucl Med 2000; Haigh PI, et al. Factors affecting sentinel node localization during preoperative breast lymphscintigraphy. 41: 1682-1688
(8) AJR 2001; Tuthill LL, et al. Biopsy of sentinel lymph nodes guided by lymphoscintigraphic mapping in patients with breast cancer. 176: 407-411
(9) J Nucl Med 2001; Yeung HW, et al. Lymphoscintigraphy and sentinel onde localization in breast cancer patients: A comparison between 1-day and 2-day protocol. 42: 420-423
(10) Radiology 2001; Birdwell RL, et al. Breast cancer. Variables affecting sentinel lymph node visualization at preoperative lymphscintigraphy. 220: 47-53
(11) Ann Surg Oncol 1999; Linehan DC, et al. Intradermal radiocolloid and intraparenchymal blue dye injection optimized sentinel node identification in breast cancer patients. 6: 450-454
(12) Ann Surg Oncol 2001; Grube BJ, Giuliano AE. Modification of the sentinel node technique: It was a hit in New York, but will it play in Poughkeepsie? 8: 3-6 (No abstract available)
(13) Ann Surg Oncol 2001; Zervos EE, et al. Localizing the sentinel node outside of the specialty center: Success of a lymphatic mapping course in disseminating new technology. 8: 7-12
(14) Ann Surg Oncol 2001; Boolbol SK, et al. Intradermal isotope injection: A highly accurate method of lymphatic mapping in breast carcinoma. 8: 20-24
(15) Ann Surg Oncol 2001; Cody HS, et al. Complementarity of blue dye and isotope sentinel node localization for breast cancer: Univariate and multivariate analysis of 966 procedures. 8: 13-19
(16) Society of Nuclear Medicine Annual Meeting Handout Book 2001; Keshtgar MRS, et al. The sentinel node in breast carcinoma- present controversies. 80-92 (No abstract available)
(17) Eur J Nucl Med 2001; Nieweg OE, et al. Summary of the second international sentinel node conference. 28: 646-649 (No abstract available)
(18) J Nucl Med 2001; Mariani G, et al. Radioguided sentinel lymph node biopsy in breast cancer surgery. 42. 1198-1215
(19) Radiographics 2002; Eubank WB, et al. Detection of locoregional and distant recurrences in breast cancer patients using FDG PET. 22: 5-17
(20) AJR 2002; Moshiri M, et al. Using lymphoscintigraphy to evaluate suspected lymphedema of the extremities. 178: 405-412 (No abstract available)
(21) Radiographics 2009; Intenzo CM, et al. Lymphoscintigraphy in cutaneous melanoma: an updated total body atlas of senitnel node mapping. 29: 1125-1135
(22) J Nucl Med 2003; Kumar R, et al. Retrospective analysis of sentinel node localization in multifocal, multicentric, palpable or nonpalpable breast cancer. 44: 7-10
(23) J Nucl Med 2003; Szuba A, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. 44: 43-57
(24) Radiology 2003; Liberman L. Lymphoscintigraphy for lymphatic mapping breast carcinoma. 228: 313-315
(25) Radiology 2003; Tanis PJ, et al. Lymphatic mapping in patients with breast carcinoma: reproducibility of lymphoscintigraphic results. 228: 546-551
(26) J Nucl Med 2003; Brenot-Rossi I, et al. Nonvisualization of axillary sentinel node during lymphoscintigraphy: is there a pathologic significance in breast cancer? 44: 1232-1237
(27) J Nucl Med 2003; Krynyckyi BR, et al. Factors affecting visualization of internal mammary sentinel nodes during lymphoscintigraphy. 44: 1387-1393
(28) Radiographics 2004; Krynyckyi BR, et al. Clinical breast lymphoscintigraphy: optimal techniques for performing studies, image atlas, and analysis of images. 24: 121-145
(29) J Nucl Med 2004; Pelosi E, et al. Sentinel lymph node detection in patients with early-stage breast cancer: comparison of periareolar and subdermal/peritumoral injection techniques. 45: 220-225
(30) N Engl J Med 2003; Veronesi U, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. 349: 546-553
(31) J Nucl Med 2005; Classe JM, et al. Prospective comparison of 3 γ-probes for sentinel lymph node detection in 200 breast cancer patients. 46: 395-399
(32) J Nucl Med 2006; Yren RF. Sentinel node biopsy in melanoma. 47: 191-195
(33) J Nucl Med 2006; Belhocine TZ, et al. Role of nuclear medicine in the management of cutaneous malignant melanoma. 47: 957-967
(34) J Nucl Med 2006; Pandit-Taskar N, et al. Organ and fetal absorbed dose estimates from 99mTc-sulfur colloid lymphoscintigraphy and sentinal node localization in breast cancer patients. 47: 1202-1208
(35) J Nucl Med 2009; Leijte JAP, et al. Visualization of tumor blockage and rerouting of lymphatic drainage in penile cancer patients by use of SPECT/CT. 50: 364-367
(36) J Nucl Med 2009; Lee JH, et al. The role of radiotracer imaging in the diagnosis and management of patients with breast cancer: part I- overview, detection, and staging. 50: 569-581
(37) J Nucl Med 2009; Vermeeren L, et al. Vaule of SPECT/CT for detection and anatomic localization of sentinel lymph nodes before laparoscopic sentinel node lymphadenectomy in prostate cancer. 50: 865-870
(38) J Nucl Med 2011; Hindie E, et al. The sentinel node
procedure in breast cancer: nuclear medicine as the starting
point. 52: 405-414
(39) AJR 2011; Burnand KM, et al. Popliteal node visualization
during standard pedal lymphscintigraphy for a swollen limb
indicates impaired lymph drainage. 197: 1443-1448
(40) J Nucl Med 2012; Buckle T, et al. Relationship between
intraprostatic tracer deposits and sentinel lymph node mapping in
prostate cancer patients. 53: 1026-1033
(41) J Nucl Med 2012; Brouwer OR, et al. Comparing the hydrid
fluorescent-radioactive tracer indocyanin green-99mTc-nanocolloid
with 99mTc-nanocolloid for sentinel node
identification: a validation study using lymphoscintigraphy and
SPECT/CT. 53: 1034-1040
(42) J Nucl Med 2012; Vidal M, et al. Accuracy and
reproducibility of lymphoscintigraphy for sentinel node detection
in patients with cutaneous melanoma. 53: 1193-1199
(43) JAMA 2012; Stoffels I, et al. Association between sentinel
node excision with or without preoperative SPECT/CT and metastatic
node detection and disease-free survival in melanoma. 308:
1007-1014
(44) JAMA 2013; Boughey JC, et al. Sentinel lymph node surgery
after neoadjuvant chemotherapy in patients with node-positive
breast cancer. The ACPSPG Z1071 (Alliance) clinical trial. 310:
1455-1461
(45) Radiographics 2013; Ecanow JS, et al. Axillary staging of
breast cancer: what every radiologist should know. 33: 1589-1612
(46) J Nucl Med 2014; Rousseau C, et al. Laparoscopic sentinel
lymph node versus hyperextensive pelvic dissection for staging
clinically localized prostate carcinoma: a prospective study of
200 patients. 55: 753-758
(47) Radiographics 2014; Humphrey KL, et al. To do or not to do:
axillary nodal evaluation after ACOSOG Z0011 trial. 34: 1807-1816
(48) 99mTc SPECT/CT versus planar lymphoscintigraphy
for preoperative sentinel lymph node detection in cervical cancer:
a systematic review and metaanalysis. 56: 675-680
(49) J Nucl Med 2015; Moncayo VM, et al. Lymphoscintigraphy and
sentinel lymph nodes. 56: 901-907
(50) Ann Surg Oncol 2015; Baker JL, et al. Comparison of [99mTc]
tilmanocept and filtered [99mTc] sulfur
colloid for identification of SLNs in breast cancer patients. 22:
40-45
(51) J Nucl Med 2015; Jimenez-Heffernan A, et al. Results of a
prospective multicenter international atomic energy agency
sentinel node trial on the value of SPECT/CT over planar imaging
in various malignancies. 56: 1338-1344
(52) Eur J Nucl Med Mol Imaging 2013; Giammarile F, et al. The
EANM and SNMMI practice guidelines for lymphoscintigraphy and
sentinel node localization. 40: 1932-1947
(53) Radiographics 2017; Cahoon AR, et al. Internal thoracic
lymphadenopathy in breast cancer. 37: 1024-1036
(54) J Nucl Med 2020; Hinsenveld FJ, et al. Prostate-specific
membrane antigen PET/CT combined with sentinel node biopsy for
primary node staging in prostate cancer. 61: 540-545
(55) Radiology 2020; Kim WH, et al. Axillary node burden assessed
with pretreatment breast MRI is associated with failed sentinel
lymph node identification after neoadjuvant chemotherapy for
breast cancer. 295: 275-282