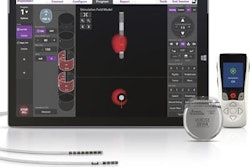
Interventional and ultrasound technology developer Boston Scientific has received U.S. Food and Drug Administration (FDA) clearance for a suite of pacemakers deemed conditional for use in the MRI environment.
The ImageReady MR-conditional pacing system, which includes the Accolade MRI and Essentio MRI pacemakers as well as the new Ingevity MRI pacing leads, is designed to treat bradycardia. Patients implanted with the full system are able to receive full-body MR scans in 1.5-tesla environments when conditions of use are met.
The Ingevity MRI pacing leads include active and passive fixation models; the FDA's move marks the first time a passive fixation pacing lead is approved for U.S. patients undergoing MR scans, Boston Scientific said.
The company is also actively pursuing MRI compatibility for its implanted cardiac defibrillation (ICD) and cardiac resynchronization therapy (CRT) systems via the global ENABLE MRI study, which was initiated earlier this year. Trial findings will be submitted to regulatory agencies in Asia and the U.S. when the company requests updated labeling for MR-conditional use on ICD and CRT systems, including those that have been previously implanted.



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)











