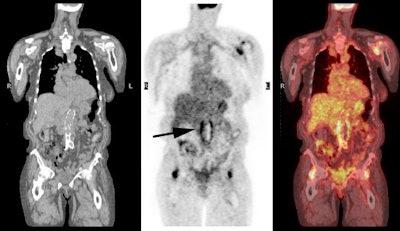
PET Oncologic Imaging:
[18]F-fluorodeoxyglucose (FDG)
Imaging:
Basic principles:
The transport of glucose into a cell is mediated by a family of
structurally related glucose transporter proteins [23]. One of the
biochemical characteristics of malignant cells in an enhanced rate
of glucose metabolism due to increased number of these cell
surface glucose transporter proteins (primarily Glut-1 and Glut-3
that are hypoxia responsive) and increased intracellular enzyme
levels of hexokinase and phosphofructokinase which promote
glycolysis [4,16,20,207]. Cancer cells can increase glucose
metabolism and preferentially utilize glucose even in the presence
of oxygen [326]. This enhanced glycolytic rate of malignant cells
facilitates their detection utilizing PET FDG imaging. Increased
glycolysis also leads to elevated levels of lactic acid (an
acidotic environment) [326]. The resultant acidotic and hypoxic
tumor microenvironment activates hypoxia-inducible factors that
promote tumor cell proliferation and angiogenesis [326]. Rapid
proliferation, poor lymphatic drainage, and increased
extracellular matrix production all contribute to increased
interstitial fluid pressure in the tumor microenvironment that can
impede effective delivery of chemotherapeutic drugs [326].
The Warburg effect represents
metabolic reprogramming of cancer cells to favor glycolytic
metabolism [337]. Several processes determine FDG uptake in tumor
cells [28]. Of major importance is the integrity of the vascular
network that is necessary for supply of nutrients to the cell
[28]. With an intact vascular supply, FDG enters the tumor cells
by using the same facilitated transport mechanism as glucose (via
cell surface proteins). The most common glucose transport protein
over-expressed on the tumor cell membranes is Glut-1, which is
insulin independent [28]. In-vitro studies have shown that FDG
uptake is also determined by the number of viable tumor cells
within a lesion (tumor-cell density) [16, 28]. Non-tumoral tissue
such as necrotic and fibrotic tissue may reduce tracer uptake
[16]. Increased cell proliferation in tumors (assessed by the
mitotic rate) also results in increased glucose utilization [28].
Tumor hypoxia will also increase FDG uptake through
hypoxia-inducible factor-1-alfa that up-regulates Glut-1 receptors
[28]. The bottom line is that FDG accumulation within a tumor is
likely related to a complex interaction between the cellular
energy demand and the tumoral microenvironment [7].
Once inside the cell, FDG is
phosphorylated by hexokinase into FDG-6-phosphate.
FDG-6-phosphate does not enter into further metabolism and
accumulates intracellularly [4]. Reduced levels of
glucose-6-phosphatase (an enzyme which metabolizes
FDG-6-phospahte) within tumor cells compared to normal and
inflammatory cells permits longer intracellular retention of
FDG-6-phosphate [22,110,207]. The signal derived from tumors
represents an average of the FDG uptake throughout the lesion
[16].
Unfortunately, FDG is not a cancer specific agent and its uptake has been described in a number of inflammatory lesions including sarcoid, tuberculosis, fungal infection, and cerebral abscess [1,20]. The increased accumulation is probably related to a markedly increased rate of glycolysis within activated inflammatory cells. Delayed or dual phase imaging may help to differentiate between benign and malignant processes [27]. A persistent or increased level of FDG accumulation within a lesion on delayed imaging is indicative of a malignant process [27].
Patient preparation:
FDG imaging is performed in the fasting state to minimize competitive inhibition of FDG uptake by glucose [14]. A minimum 4 hour fast is recommended prior to initiation of the PET FDG study. A 12 hour fast may decrease accumulation by the myocardium and improve detection of mediastinal metastases in cases of lung or breast cancer. Patients with early morning exams should not eat anything after midnight. Patients with afternoon exams should eat a light breakfast with minimal carbohydrate containing foods [143]. Patients should be well hydrated for the exam (while fasting patients should try to drink at least two to three 12 ounce glasses of water) and should avoid strenuous work or exercise for 24 hours before scanning [73,143]. Some recommend that patients also be on a low carbohydrate diet for 24 hours prior to the study [143]. For head and neck cancers, use of a benzodiazepine can aid in decreasing neck muscle activity [143]. Some authors recommend the use of a high-fat, low-carbohydrate, protein-preferred meal the night before the scan and a vegetable oil drink on the morning of the scan to more effectively suppress cardiac activity [199,202]. Using this protocol, the increased free fatty acid availability promotes FFA oxidation and inhibits glucose use [199,202]. This protocol may also aid in decreasing the incidence of brown fat activity compared to fasting patients (2.8% versus 6.3% of the patients, respectively in one study) [202].
If there is a possibility of an elevated serum glucose level, a
serum glucose level is obtained prior to FDG administration. FDG
uptake is significantly influenced by plasma glucose levels and
uptake will be decreased when plasma glucose levels are elevated
(elevated serum glucose levels can result in decreased FDG
accumulation within the tumors) [4,14].
If the glucose level is higher than 150-200 mg/dL (8.3-11.1
mmol/L), the study should be delayed until the glucose level is
under 200 mg/dL [3,76,195]. Although
less well documented than acute hyperglycemia, chromic
hyperglycemia is assumed to have a smaller negative influence on
tumor uptake and tracer distribution [195]. Administering insulin
at the same time as FDG should be avoided because it tends to
increase accumulation in skeletal muscle and thus less FDG is
available for accumulation in tumors [3]. In patients with
diabetes, blood sugar control should be achieved with oral
hypoglycemic agents or insulin (not administered near the time of
FDG administration) [3]. Diabetic patients are best imaged early
in the morning before the first meal and insulin or oral
hypoglycemic medication should be titrated appropriately the night
before and morning of the study [143]. Subjects with type I or II
diabetes who cannot reliably attain acceptable glucose levels
early in the morning should be scheduled for late morning imaging,
should eat a normal breakfast at 7 AM, should take their normal
morning diabetic drugs, and then should fast for at least 4 hours
before the PET exam [300]. If possible, diabetic patients
should test their ability to maintain reasonable glucose levels
after fasting prior to the PET exam [143]. If required, some
authors have found that insulin can be administered, but a waiting
period of at least 90 minutes should be observed prior to
administration of the FDG [195]. Even then, about 25% of patients
will have unacceptable biodistribution of FDG and about 10% of
patients can develop hypoglycemia [195].
|
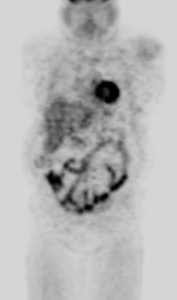
Hyperinsulinemia: The patient below had
a normal glucose level and was injected for an FDG PET
scan. Imaging revealed intense cardiac uptake and a large
about of muscular activity. The findings are consistent
with a hyperinsulinemic state and the patient subsequently
admitted to eating a small breakfast. |
|
|
For specific imaging of the kidneys or pelvic region additional preparation can be performed. A foley catheter in the bladder will aid in reducing urine activity [143]. Administration of a diuretic (lasix 20-40 mg) 10-15 minutes after FDG administration and when possible IV hydration with 250-500 mL of saline (not dextrose containing solutions) will also aid in dilution and clearance of urinary activity [143].
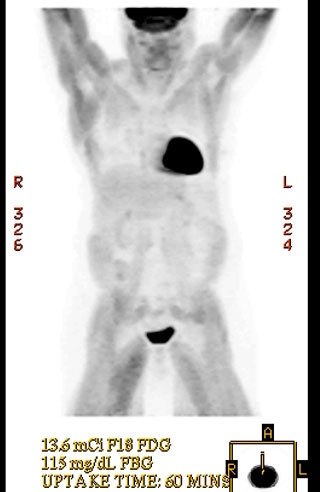
For oncologic imaging, FDG-PET scans are performed approximately 60 minutes following the intravenous injection of 10-20 mCi of FDG (0.14-0.21 mCi/kg of body weight) [143]. For pediatric use, a dose of 0.15-0.30 mCi/kg has been recommended with a minimum dose of 1 mCi [125]. A normal PET acquisition is acquired from the base of the brain to the mid thighs [3]. For patients with melanoma, a whole body acquisition is performed from the top of the skull to the feet [235]. Some authors recommend that entire body imaging should be considered in all patients as unsuspected sites of malignancy may be found outside the normally scanned area in up to 4% of patients [235]. The standard PET exam previously required 45 minutes to 1 hour to acquire when performing attenuation corrected exams using a conventional BGO scanner [15]. LSO PET cameras and modern PET/CT scannsers can perform a similar 3D acquisition in a much shorter period of time.
In addition to standard body images, some centers acquire a 4-minute brain scan about 30 minutes following FDG administration using the three-dimensional acquisition mode [3]. However, routine CNS imaging is not universally performed [45]. The normal brain has substantial glucose uptake and metastatic foci may be difficult to detect on PET FDG images [42,43,44,45].
Overall efficacy:
PET/CT has been shown to be significantly more accurate (86%) in tumor staging compared to CT alone (63%) [235]. The results of a large number of studies has shown that the results of FDG PET imaging can have a major impact on oncologic patient management. Up to 15% of patients without clinical suspicion of recurrence or residual disease are found to have active tumor on PET imaging [47]. PET exam findings can change planned radiation therapy (dose, volume, or intent) in 27% of patients [47]. PET imaging can also detect unexpected sites of secondary malignancy (in 1.2 to 2% of patients) [75,113]. The identification of unexpected hypermetabolic foci on FDG PET imaging should prompt further evaluation as up to 71% of these foci can be malignant or premalignant [75]. Unfortunately, not all tumors will shown significant metabolic activity on FDG PET imaging [47]. PET exams are positive for tumor in about three-quarters of patients with known primary or residual tumor [47].
One other drawback of PET imaging is the underestimation of metabolic activity in tumors that are smaller than two times the spatial resolution of the scanner (partial volume effect) [6,118]. For a spherical lesion with a diameter 1.5 times the spatial resolution of the PET scanner at full width at half maximum, the measured maximum activity concentration is only 60% of the true activity (and the measured mean activity concentration on 30% of the true activity) [118]. Despite this limitation, using modern PET scanners, objects that measure as small as 5 mm can be visualized [1]. Assessment for malignancy and metastatic disease with PET FDG imaging is improved when supplemented by CT images [2]. PET imaging can also identify a more accessible tumor site for biopsy.
In cancer screening of asymptomatic individuals, FDG PET imaging can detect unsuspected malignancy in 1.1-1.2% of patients [113]. However, PET imaging is not recommended as a screening exam.
Monitoring response to therapy:
Individual tumors will vary widely in their response to a
particular form of therapy [53]. These differences are
likely multifactoral and related to disparities in tumor biology
and the presence of drug and radio-resistance mechanisms [53]. The
early identification of tumors that are not responding to
conventional therapies would permit the timely institution of
alternative treatment that may be more effective [53]. The World
Health Organization (WHO) criteria define response as a decrease
of at least 50% in the sun of the product of the longest
perpendicular diameters of measured lesions [159]. The more recent
RECIST criteria introduced by the National Cancer Institute
defines response as a 30% decrease in the largest diameter of a
tumor (for a spherical lesion this value is equivalent to a 50%
decrease in the product of 2 diameters [159]. Unfortunately,
conventional imaging modalities are somewhat limited in their
ability to evaluate for treatment response as residual masses may
persist on imaging studies despite resolution of disease activity
[53]. In other words- anatomic changes often lag behind tumor cell
mortality [53]. FDG PET imaging can evaluate tumor response to
therapy before anatomic changes are observed [53]. PET imaging is
currently the most sensitive and specific imaging method to obtain
information about tumor physiology [53]. Decreased tumor FDG
uptake following initiation of treatment is a complex biologic
process linked to a decline in the number of viable tumor cells,
reduction of the proliferative activity of tumors, and changes in
glucose metabolism of viable tumor cells [196]. Studies indicate
that a measurable decrease in tumor FDG uptake 1-3 weeks following
chemotherapy is associated with effective treatment [118].
Therefore, post-treatment imaging should be performed 2 weeks
following the end of a specific chemotherapy cycle, while waiting
6-8 weeks following radiation therapy [143]. Patients who do not
demonstrate a change in FDG uptake should be considered for a
change in treatment regimen [118]. By providing a more timely and
accurate assessment of treatment efficacy, PET imaging can have
significant impact on clinical treatment decisions [53].
For proper interpretation of tumor response, it is important to have a baseline examination [53]. Imaging 10 days to 2 weeks after completion of chemotherapy is recommended to avoid transient fluctuations in FDG metabolism (tumor stunning) [53,158]. For radiation therapy a longer delay may be required (up to 60 days) prior to imaging. Generally, FDG uptake 6 months after radiotherapy is associated with tumor recurrence [53]. Assessment of tumor response to therapy can be determined by visual assessment of the images or by quantitative analysis using standard uptake values [53].
Standardized uptake ratio (SUR) or standardized uptake value (SUV):
A standardized uptake value (SUV), is used to determine if a lesion has increased 18FDG activity. The SUV normalizes the amount of FDG accumulation in a region of interest (ROI) to the total injected dose and the patient?s body weight in kilograms [60]. It provides a means of comparison of FDG uptake between patients [14]. The SUV is calculated by dividing the mean activity within a selected region (or volume) of interest (in mCi/ml) by the injected dose (in mCi/kg). Modifications of the SUV that may improve the semiquantitative evaluation of FDG uptake include using body surface area or the lean body weight instead of the weight of the patient; this is significant because the distribution of FDG is higher in muscle than in fat [4]. Specifically, in pediatric patients an SUV calculation based upon body surface area seems to be a more uniform parameter than SUV determined on body weight [125].
SUVmean= Average selected
region activity (mCi/ml)
Injected dose (mCi)/body weight (kg)
SUVmean can vary depending on the size of the region or volume of
interest [254]. Because of this variability, there is not a
consistent manner allowing SUVmean to be compared across sites and
SUVmax has become the standard in quantification for PET studies
[254].
The SUVmax is defined as the SUV derived from the single voxel
showing the highest uptake within a defined ROI or VOI [254]. The
SUVmax typically represents the most metabolically active part of
the tumor and it is nearly free from observer variability and is
less susceptible to partial volume effects [254,260]. A drawback
of SUVmax is that because it is derived from a single voxel, it
may not be an adequate surrogate marker for true tumor biology and
it can be heavily influenced by image noise and voxel size
[254,258,306]. Additionally, motion can result in blurring of the
target volume resulting in a reduction in the measured SUVmax
[306].
An SUVmax greater than 2.5 has been shown to be very sensitive
and specific for malignant lesions [37]. Visual analysis of the
amount of uptake within a lesion has also been shown to be
effective in differentiating benign from malignant lesions [37,38]. Uptake greater than blood pool
activity (i.e.: the liver and mediastium typically have SUV's of
about 2.0) indicates a malignant lesion, while activity equal to
or less than mediastinal blood pool suggests a benign lesion [38].
In fact, visual analysis may be more sensitive for nodules smaller
than 1.5 cm in size (although, the improved sensitivity comes at a
decreased specificity) [38].
It is important to remember that SUV values may change with time after FDG injection; thus, the time of acquisition after FDG injection must be standardized for the values to be useful [3]. Other factors that can affect the SUV include patient motion (due to lesion blurring), the blood glucose level at the time of injection, and partial-volume effects (scanner resolution) [16, 36]. Partial volume effects represent underestimation of metabolic activity in tumors that are smaller than two times the spatial resolution of the scanner [6]. An accurate SUV determination is more difficult for nodules smaller than 1.5 cm due to partial volume effects [85].
Factors affecting SUV measurement: (See also PET/CT discussion for the effects of contrast on SUV measurement)
- Plasma glucose
levels: High plasma glucose can decrease FDG uptake by
competitive inhibition [111] and this will decrease the
calculated SUV [110]. If the patients
blood sugar is above 200 mg/dL the study should be postponed
[111]. Chronic blood sugar elevation as seen in diabetic
patients seems to have a less significant effect on tumor FDG
uptake [111]. However, diabetic patients should have proper
blood sugar control prior to PET imaging [111]. It is also
important to note that insulin injection close to the time of
FDG administration will increase skeletal muscle uptake of FDG
and this can degrade scan quality by reducing the
lesion-to-background ratio [111].
- Time after
administration that SUV is measured- FDG uptake in malignant
tumors is time dependent and often increases for up to 90
minutes or longer after tracer injection [118,128]. SUV should
be measured at a fixed time following injection to ensure
accuracy.
- Subcutaneous
infiltration of the tracer: SUV is based upon the injected
dose. Infiltration of the tracer will result in an error
because of the decrease in the real dose available for
distribution [110,118]. Incorrect measurement of the injected
amount because of residual tracer in the syringe can also
result in incorrect SUV measurements [306].
- Body weight or body
surface area- FDG distribution is weight dependent- heavier
patients often have a body fat higher percentage and fat is
less metabolically active than muscle tissue- hence, the SUV
in obese patients is increased compared to thin patients
[226,293]. A thin patient with relatively more muscle will
likely have a lower SUV for a given lesion because muscle
competes for the FDG [226]. Even SUV comparison between
examinations of the same patient can be flawed if the patient
has lost or gained weight [226]. The use of a lean body mass
determination for SUV measurement in obese patients has been
found to be a better representation of metabolic activity
[293,306]. Lean body mass is calculated in male subjects as
1.10 x weight - 128 x (weight2/height2)
and in females as 1.07 x weight - 148 x (weight2/height2), where
weight is measured in kg and height in cm [306].
- Size of the region
of interest- because of partial volume effects, the measured
mean tumor uptake will decrease when the size of the ROI used
to define the tumor is increased [118].
- Resolution of the
scanner- image resolution affects the measured SUV of a small
object because of partial volume effects [226].
- Type of image reconstruction and attenuation correction: SUV measurements from filtered back projection (FBP) images underestimate the true activity concentration by about 20%- this is much greater than iterative reconstruction (IR) images which underestimate activity concentration by about 5% [83]. The discrepancy is primarily related to the way transmission data is processed- measured attenuation correction for FBP images and segmented attenuation correction for IR images [83]. The number of iterations used for ordered-subsets expectation maximization (OSEM) reconstruction can also affect the measured SUV value [107]. For max SUV the value increases systematically with a 28% increase from 5 to 40 iterations [107]. The same field-of-view and reconstruction parameters should be used for all followup studies [226].
The PERCIST response criteria suggest the use of a 1 cm3 ROI surrounding the voxel with the highest activity [306]. The mean value of the radiotracer activity within that ROI (SUVpeak) is then normalized to lean body mass and reported as peak SUL (SULpeak) [306]. The disadvantage of SULpeak is difficulty with the evaluation of small lesions and the need for specialized software [306]. For background activity, a 3 cm diameter spherical VOI is placed in the right side of the liver, midway between the dome and the inferior margin, excluding central ducts and vessels [310]. For a tumor to be measurable at baseline, the SULpeak must be greater than or equal to 1.5 times the mean SUL in the 3 cm diameter spherical VOI plus two times its standard deviation to have a minimum threshold for evaluation [310]. For follow up scans, the difference between the injection to imaging time between the baseline and followup study should be less than or equal to 15 minutes [310]. The injection to beginning of imaging should be greater than or equal to 50 minutes and less than or equal to 70 minutes [310]. Serum glucose levels must be less than 200 mg/dL [310].
PERCIST 1.0 criteria for response assessement:
- Complete metabolic response: Visual disappearance of all metabolically active tumor [310].
- Partial response: SULpeak decrease by 30% or more (between the most intense lesion at baseline and the most intense lesion at follow-up (not necessarily the same lesion). There should be no new lesions in a pattern suspicious for cancer and no increase in size greater than 30% in the target or non-target lesions [310].
- Stable disease: SUL peak decrease of less than 30% or increase of 30% or less
- Progressive disease: SULpeak increase of more than 30%, new lesions, or a lesion increase in size of greater than or equal to 30% [310].
A tumor-to-blood standardized uptake ratio (SUR= tumor SUV/blood SUV) may also be used to improve the prognostic value [332]. The SUR can also be corrected for uptake time variations by converting the measured uptake values to a preselected fixed scanning time point [332]. This scan time normalized SUR removes several shortcomings of SUVmax and results in decreased test-retest variability [332].
Three dimensional PET indices such as metabolic tumor volume and total lesion glycolysis have also been developed and may provide better prognostic information by providing a more accurate reflection of the total tumor burden [265]. Total lesion glycolysis (TLG) integrates both anatomic and biologic data and may also be useful for prediction of prognosis [256]. TLG is defined as the SUVmean multiplied by the metabolic tumor volume (MTV) [256]. The MTV represents the metabolically active portion of a mass as delineated by a specified threshold of the maximum SUV (above a minimum threshold). There is no standard definition for the metabolic tumor volume and various measures can be used to define an isocontour (for instance- the metabolic tumor volume could be defined by all voxels with an SUV above 2.5 or above a threshold of 41-42% of the SUVmax) [256,265,317]. However, use of 41-42% of SUVmax to determine MTV and TLG will underestimate lesion uptake with a high activity and overestimate lesions with an SUVmax close to background level [308]. Adaptive methods based on signal-to-background ratio have also been developed [332]. This is similar to the fixed threshold method, except that it adapts the threshold relative to the local average background activity, thereby correcting for the contrast between the tumor and local background.
The measurement of tumor texture/heterogeneity (corresponding to necrosis, fibrosis, and areas with higher cellular proliferation) has also been evaluated for prediction of tumor response to therapy [287]. Increased tumor heterogeneity often associated with adverse tumor biology, increased lesion aggressiveness and greater risk for treatment failure [287,291,301]. Exactly how tumor heterogeneity should be measured is not entirely clear [287]. Additionally, image reconstruction features (iteration number, grid size, FWHM of the gaussian filter) impact on the image features and this can affect heterogeneity/texture features [301].
Determining
metabolic response:
Changes in the SUV measurement also play a role in evaluating
tumor response to therapy [126,226]. Studies have suggested that
SUV has an in subject variation of approximately 10% [319]. A
change of more than 20% is generally considered outside the range
of spontaneous fluctuation and to represent a true change in the
glucose metabolism of the tumor mass [126]. Studies indicate that
most tumors responding to treatment show a 20-40% decrease in SUV
early in the treatment course [226]. An important point to
remember is that with current technology, PET can measure the
first two logs of tumor cell kill (i.e.: a negative PET
result does not indicate a total eradication of disease, but it
does imply a certain amount of cell killing (between 2 and 10 log
units of tumor cell killing) [158]) [281]. Thus- a negative
post-treatment PET scan at the end of therapy either means there
are no cancer cells present or that there are fewer than 107
cells [281]. Therefore, although a completely negative PET at the
end of therapy is suggestive of a good prognosis, it does not
necessarily correspond with compete eradication of cancer cells
[281]. Thus, patients with negative mid or post treatment scans
represent a heterogeneous group in terms of relapse risk [158]. A
negative mid-treatment scans may imply a greater likelihood for
cure as these patients will receive additional chemotherapy which
will result in additional cell killing [158].
The European Organization for Research and Treatment of Cancer
determines treatment response depending on the percentage change
in SUVmax- % change SUVmax = (F/U - Baseline)/ Baseline x 100
[281]. They recommends that an increase in SUV measurement of 25%
from the baseline scan or the appearance of new FDG positive
lesions be classified as progressive disease, while a partial
response is represented by a 15-25% decrease after one cycle of
chemotherapy and greater than 25% after more than one treatment
cycle [131,281]. Stable disease is defined as a decrease in
activity of less than 15% or an increase in metabolic activity of
less than 25% [281]. Complete metabolic response is defined as
complete resolution of FDG uptake in the tumor [147] so that
activity is less intense than the liver and indistinguishable from
surrounding background blood pool levels [238].
PRECIST criteria (PET response criteria in solid tumors) have
also been published. For PRECIST a new metric the SUVpeak
has been developed. The SUVpeak is defined as the mean FDG uptake
within a spheric 1cm3 (other indicate a 12 mm diameter
[295]) region around the tumor voxel with the highest SUV
[283,295]. The SUVpeak is intended to provide a more reproducible
parameter of maximal lesion uptake [283]. A CMR is defined as
complete resolution of tumor uptake, a partial metabolic responder
demonstrates a minimum reduction of 30% in a lesion (or 0.8 SUV
units in absolute terms), progressive metabolic disease as an
increase of a minimum of 30% in a lesion or development of new
lesions, and stable metabolic disease as anything that does not
meet the above criteria [252,295]. PRECIST defines a 3 cm
spherical ROI in the right hepatic lobe as a QA tool to
assess the applicability of quantitative comparisons [296]. If the
liver or blood pool is to be used for determination of
tumor-to-background ratios to monitor therapy response, these
values can vary between exams (in one study normal variability in
SUV mean for blood pool was -0.8 to 0.9 and for the liver was -0.9
to 1.1 [277,296].
Additionally, in the presence of intense hypermetabolic tumors
and/or bulky (extensive) neoplastic disease, the energy substrate
requirements of the tumor may create a "reservoir" or "sink"
effect which results in decreased radiopharmaceutical available
for uptake in other organs [329]. This can affect the activity in
the liver or blood pool (and brain) which are often used for
semi-quantitative assessment of metabolic tumor therapy response
[329]. Normalizing to a reference tissue, such as the blood, can
remove this effect [329].
In patients with solid tumors treated by pre-surgical chemotherapy, a change in FDG uptake of 30-35% within the first few weeks of chemotherapy has been found to provide the highest accuracy for the prediction of histopatholgic complete or subtotal tumor regression [159]. Although there is interobserver variability in SUV max measurements, this variability has been shown to be significantly lower than the variability in CT size measurements for tumor response [206]. Variability in SUV max tends to be greater for treated tumors because of the challenge in selecting tumor ROI's when there is little FDG uptake above background tissues that results in greater statistical noise [206].
PET in Radiation Planning: See also individual tumors
The results of the PET examination can also be incorporated into
radiation planning volumes [82]. However, there are several
factors which affect PET images that can influence target edges or
margins including partial volume effects (tumor size), patient
motion, image resolution, heterogeneity of tracer uptake within
the tumor, and window display level [82,253]. Most commonly, the
tumor volume is delineated using a fixed percentage of the maximum
FDG concentration/maximum voxel value (i.e.-
50% isocontours) [211]. Manually defined tumor volumes may be the
most accurate way to delineate the tumor, but suffers from large
intra- and interobserver variability and is time consuming [253].
PET in Radiofrequency ablation evaluation: See also individual tumors
Following radiofrequency ablation, activity within the tumor decreases to background levels as soon as one day following the treatment [145]. However, a ring-shaped area of increased activity around the site of the tumor can be seen due to inflammatory changes surrounding the zone of necrosis [145]. This activity will decrease in intensity over time, but PET imaging may need to be delayed 6-8 weeks following RF treatment to better evaluate for treatment response [145].
The normal distribution for FDG includes:
1- Head and neck:
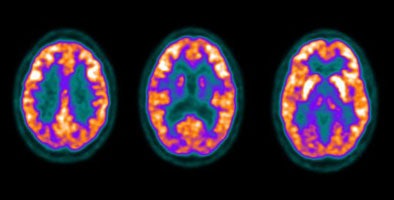
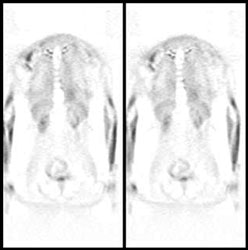
The cortex of the brain- there is generally very intense tracer uptake in the brain because the brains only energy source is glucose. The total uptake in the brain is approximately 6% of the injected dose [20]. Metastatic CNS lesions generally have activity similar to gray matter [24]. During whole body scanning for the evaluation of patients with primary malignancy, unsuspected CNS metastases are detected in less than 1% of patients [24]. Some metastases may demonstrate decreased activity compared to normal brain (possibly related to edema) [45]. For the identification of cerebral metastases FDG PET has a sensitivity of 75% and a specificity of 83% [45]. Between 32% to 40% of cerebral metastases seen on MR imaging will not be detected on FDG PET exams [45,46]. Lesion size is an important determinant in the ability to identify a CNS lesion of PET [45]. The likelihood of detecting a 1 cm size metastatic CNS lesion is only about 40%, while a 1.8 cm lesion has a mean detection rate of about 90% [45]. It has been found that when scanning of the brain is included for oncologic FDG PET imaging a change in treatment occurs in fewer than 1% of patients [45]. Because FDG PET imaging detects few clinically relavent lesions in patients with primary malignancies, it should not be performed routinely [24].
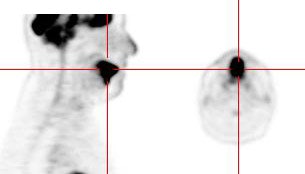
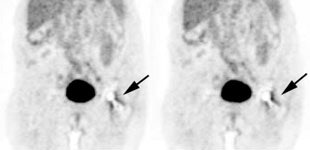
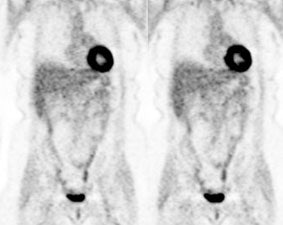
Focal incidental uptake in the pituitary gland can be seen in about 0.8% of patients and should prompt further evaluation as an underlying abnormality can be found in up to 41% of cases- most commonly pituitary adenomas [244]. Using a cutoff SUVmax of 4.1 may be helpful in distinguishing pathologic uptake from physiologic uptake [244].
|
Normal brain activity: The image below demonstrates normal FDG brain activity. |
|
|
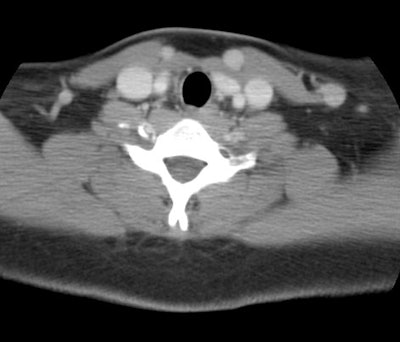
The tonsils are constantly exposed to antigens causing carious
degrees of physiologic inflammation [220]. Low to moderate FDG
uptake occurs in the lingual and palatine tonsils and at the base
of the tongue because of physiologic activity associated with the
lymphatic tissue in Waldeyer's ring [41]. However, tonsil uptake
can be high (SUV 3.11 (lingual) to 3.48 (palatine)) [103],
however, physiologic uptake is typically symmetric and asymmetric
uptake should be regarded as suspicious for malignancy [220].
There is usually uptake in the lymphoid tissue of Waldeyer's ring
[4]. The soft palate can also show tracer uptake [103]. Variable,
but typically low, uptake can be seen in the salivary glands which
secrete low amounts of glucose [41]. The parotids glands also show
mild, symmetric tracer uptake. Due to this physiologic activity,
low grade malignant lesions in the salivary and parotid glands may
be obscured [157]. Additionally, PET imaging is not able to
accurately differentiate benign from malignant parotid lesions as
benign tumors such as pleomorphic adenoma, oncocytoma, and Warthin
tumor are known to be FDG avid [312].
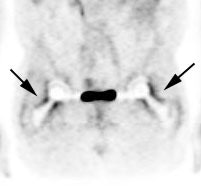
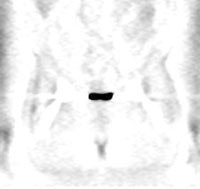
|
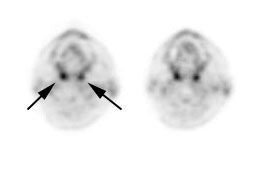
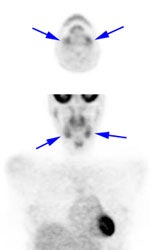
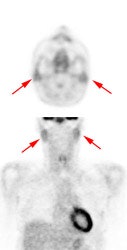
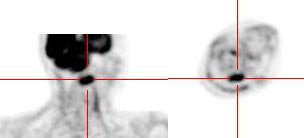
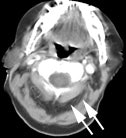
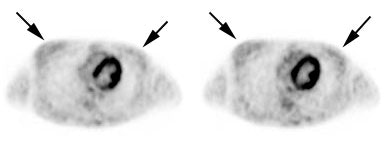
Head and neck activity: The images below show typical uptake in the tonsils (black arrows), submandibular glands (blue arrows), and parotid glands (red arrows) |
|
|
2- Myocardium- In the post-prandial state (following a meal),
plasma insulin levels increase and there is marked cardiac
activity (due to increased GLUT-1 and GLUT-2 transport activity)
[251]. Little myocardial activity is generally noted in the
fasting state as the myocardium preferentially utilizes fatty
acids for energy generation [251]. However, uptake can be variable
as even in the fasting state, glucose can still account for 30-40%
of the energy derived from oxidative metabolism [25]. Even
overnight fasting may be inadequate to reduce physiologic cardiac
FDG uptake [251]. Fasting myocardial uptake tends to be nonuniform
with a significant decrease in activity in the septum and anterior
walls compared to the lateral and posterior walls (a
posterolateral increased in cardiac FDG activity is a common
normal physiologic pattern) [251]. Focal activity in the papillary
muscles is also common [215]. This regional variation in FDG
uptake likely reflects physiologic differences in local myocardial
substrate utilization that may be related to variations in
myocardial wall stress [251].
Certain drugs may also interfere with suppression of myocardial
FDG uptake including beta-blockers and the antianginal drug
trimetazidine (a FFA oxidation inhibitor) [199]. Bezafibrate, a
drug used to treat hyperlipidemia, and levothyroxine (thyroid
hormone) taken before FDG administration have also been shown to
lower FDG cardiac uptake [251].
Some authors recommend the use of a high-fat, low-carbohydrate,
protein-preferred meal the night before the scan and a vegetable
oil drink on the morning of the scan to more effectively suppress
cardiac activity [199,202]. Using this protocol, the increased
free fatty acid availability promotes FFA oxidation and inhibits
glucose use [199,202]. The use of unfractionated heparin (4000
units 15 minutes prior to FDG administration) has also been
suggested to be effective in decreaing cardiac uptake [245].
Heparin causes the release of free fatty acids into the
circulation and this works to reduce physiologic myocardial FDG
accumulation [245]. However, the best suppression of myocardial
uptake may be seen with a low carbohydrate (<5gm) dinner in the
evening prior to the scan, and then fasting until the FDG
injection [221].
Increased FDG localization in the atria is associated with atrial
fibrillation [251]. The crista terminalis (a crescent-shaped
muscle band located at the junction of the right atrium and right
atrial appendage) may also occasionally demonstrate increased FDG
uptake [251].
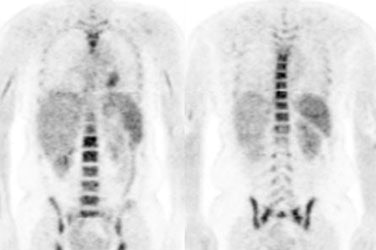
Increased FDG uptake has also been reported in lipomatous hypertrophy of the interatrial septum- mean SUV 5.6 [101]. Uptake can be seen in up to 82% of patients with LHIS [101]. The etiology for the uptake is not certain, but may be related to the presence of brown fat [101]. Fusion PET/CT helps to clarify the location of the uptake [101].
|
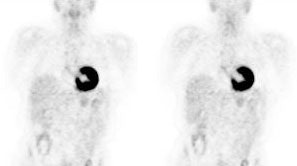
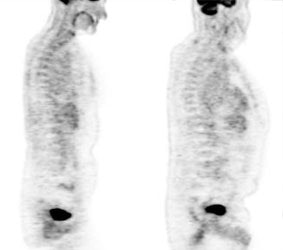
Myocardial activity: Myocardial uptake can be very variable. The patient on the left below had a glucose level of 97 prior to FDG injection. Despite the normal serum glucose, note the intense cardiac activity in this patient. The patient on the right was a diabetic patient with a blood glucose of 169- note that there is no myocardial uptake in this patient despite the elevated glucose level. |
|
|
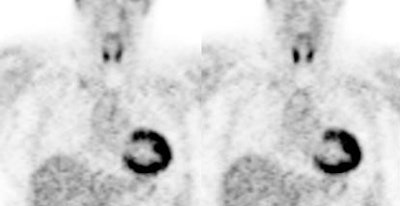
3- Renal/Urinary bladder- Unlike glucose, FDG is filtered by the glomerulus and not resorbed [54]. Hydration and frequent voiding promote diuresis and help to decrease the radiation dose to the genitourinary tract [4]. The use of IV hydration and IV lasix (0.5 mg/kg up to 40 mg with 3 successive urinary bladder voidings) can help to decrease/clear ureteral and urinary bladder activity in cases in where there are equivocal pelvic findings [153].
|
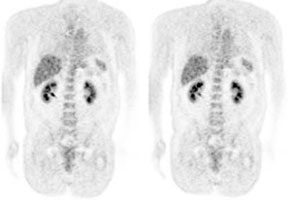
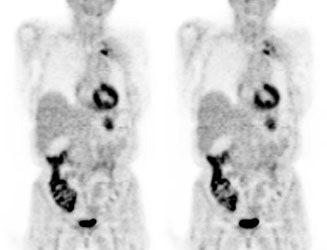
Genitourinary activity: The image below demonstrates normal renal and hepatic activity. |
|
|
|
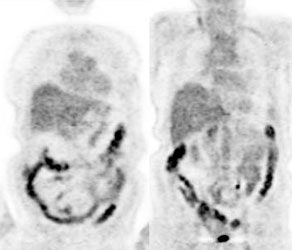
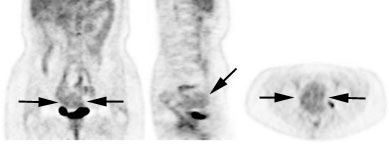
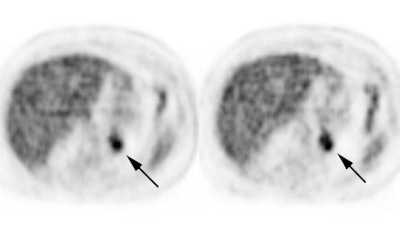
Genitourinary activity: The image below demonstrates activity within the collecting system of a transplanted kidney (black arrows). This should not be mistaken for an abnormality. |
|
|
|
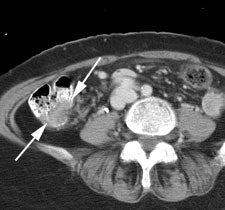
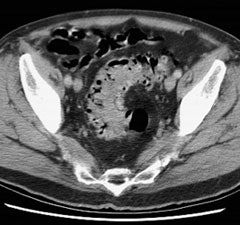
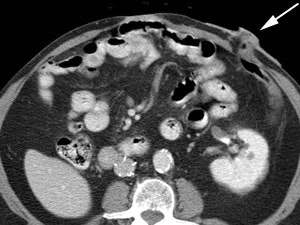
Bladder cancer: Dilute urinary activity in this patient's bladder allowed accurate detection of their bladder cancer (white arrows on CT, black arrows on PET scan). In general, urinary excretion of FDG limits evaluation of urinary tract malignancies, however, hydration and diuresis can improve diagnostic accuracy. |
|
|
4- Liver- faint, heterogeneous activity is common. It has been
reported that fatty liver changes can result in slightly decreased
metabolic activity in the liver [217,240], but other authors have
noted no significant change in SUV max between fatty and non-fatty
livers [218] and even increased FDG uptake in hepatic steatosis
has been reported and felt to be related to inflammatory cells
(possibly reflective of nonalcoholic steatohepatitis or NASH)
[272,321]. It is possible for both liver metastases and treated
liver metastases to have liver-equivalent activity- as a result,
such lesions cannot be reliably identified by PET imaging [17].
Acute cholangitis can demonstrate foci of tracer uptake along the
course of the intrahepatic ducts, but this can be difficult to
distinguish from true liver lesions [39]. Uptake can also be seen
along biliary stents [39]. In patients with malignancies, focal
uptake in the liver on PET imaging without corresponding
abnormality on CT can be associated with malignancy in more than
75% of cases and should be further evaluated with liver MRI [333].
5- Musculoskeltal and soft tissue activity:
Exercise should be avoided on the day of scanning to avoid muscle uptake. The muscles of mastication or larynx can accumulate tracer if eating or talking [4]. Asymmetric uptake of FDG can be seen in the small internal laryngeal muscles in patients with laryngeal nerve palsy contralateral to the side of the nerve dysfunction and should not be misinterpreted as pathologic [29]. In patients with COPD, intercostal muscle uptake can be seen due to excessive contraction required to facilitate expiration [111]. Hyperventilation may induce uptake in the diaphragm [54] and stress-induced muscle tension is often seen in the trapezius and paraspinal muscles [4]. Benzodiazepines may be used to decrease paraspinal and posterior cervical muscle uptake in tense patients [20]. Insulin injection just prior to FDG administration will result in increased muscle uptake [111].
|
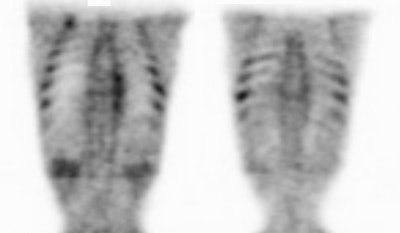
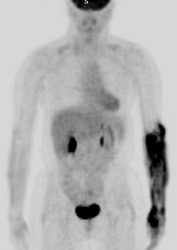
Muscle activity: Note the marked muscle uptake within the forearms in this patient. We suspect that the patient was cold or nervous and clenching there hands following injection of the tracer. |
|
|
|
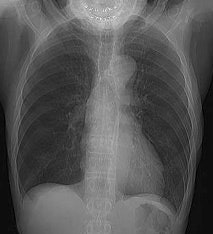
Intercostal muscle activity: Uptake appears to be within the ribs, but this actually represents uptake in the intercostal muscles in a patient with severe COPD. COPD patients use their intercostal muscles for breathing. |
|
|
A moderate amount of uptake can be seen in the anterior part of the floor of the mouth due to the genioglosus muscle which prevents the tongue from fall back in supine patients [41]. In patients that chew uptake can be seen in the masticator muscles. Speaking or coughing produces tracer uptake in larynx (pharyngeal constrictor muscles and vocal cords larynx) [127]. Asymmetric muscular uptake can be seen in the inferior obliquus capitus muscle at the skull base [127].
|
Laryngeal/vocal cord activity: Likely related to talking or coughing during tracer uptake. |
|
|
|
Intense uptake in the tongue: Intense tracer uptake in the tongue in this patient was likely related to nervous movement of the tongue during the tracer uptake phase. |
|
|
|
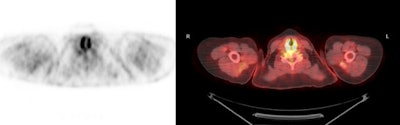
Inferior obliquus capitus muscle: The patient below presented for staging lymphoma. There was intense uptake in the posterior right neck- CT revealed this to correspond to the inferior obliquus capitus muscle [127]. The patient had torticollis which was felt to be the etiology for the asymmetric uptake in this case. |
|
|
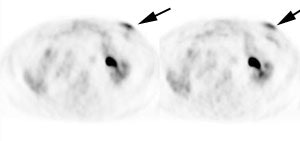
Brown fat: Adipose tissue in mammals is composed of at least two functionally different types of fat: white and brown [259]. White fat serves as an insulator and as energy storage, releases hormones and cytokines that modulate whole-body metabolism and insulin resistance [259]. Brown fat (unlike white adipose tissue fat) contains a large number of mitochondria and has the capacity to generate heat [67,173,181]. Brown fat is stimulated by several factors, including exposure to cold (decreasing body temperature) which causes over-expression of glucose transporter 4 in brown fat [62]. Brown fat can also be stimulated by ingestion of food (diet-induced thermogenesis) [259]. The incidence of FDG uptake in brown fat has been shown to be increased during periods of cooler temperatures [62,259]. Heat generation in brown fat is initiated by activation of the sympathetic nervous system (catecholamine stimulation) [173,193]. Cold stimulated FDG uptake in brown fat is also more pronounced during fasting [264].
Prominent tracer uptake has also been described within the
supraclavicular fat on PET/CT in about 2% to 5% of patients- the
etiology is not well understood, but is felt to be related to the
presence of "brown fat" (brown adipose tissue). [52,62,67,259]. Brown fat is most prominent in
newborns and diminishes with age [67]. In adults, brown fat is
significantly more common in women than men (5.9% versus 2.7%) and
is also more common in patients with lower body weight and body
mass index (the amount of brown fat in the body declines with age
and as obesity increases and brown fat activity is generally found
to be lower in older and overweight people compared to young or
leaner controls) [259,305], however, this has not been found in
all studies [323]. The incidence of tracer uptake in brown fat is
also increased in women [62,91,259] and
in pediatric patients [67].
Other areas in which brown fat uptake can less commonly be seen
include the axillae, mediastinum, intercostal paravertebral, and
perinephric regions [67,91]. Less
common locations include the posterior neck, left paratracheal
area, and retrocrural area [46]. Why brown fat persists in some
adults is not presently known- although it is possible that brown
fat may persist into adulthood more than was previously recognized
[67]. SUV max measures can be as high as 27.8 [259]. Younger
patients are more likely to have higher maximum SUV's in brown fat
[259].
Brown fat is an active metabolic organ that plays an important part in the basal metabolic rate [311]. Brown fat is known to increase glucose uptake when the sympathetic nervous system is activated by cold stimulation and other causes [86]. Beta-adrenergic agonists increase fat oxidation and thermogenesis/brown fat activity [273]. Because brown fat is stimulated by the sympathetic nervous system and pretreatment with propranolol has been shown to reduce FDG uptake in an animal model [86]. Administration of fentanyl, diazepam, or the use of a low high-fat, low-carbohydrate, and protein permitted diet may also reduce FDG uptake in brown fat [205]. Nicotine has also been shown to increased brown fat activity in animals and this effect is increased when combined with ephedrine [168] and should be avoided prior to FDG administration [183]. The Hounsfield density measurements of active brown fat have been shown to be slightly higher than non-activated area of brown fat [213]. There is a negative correlation between brown fat and coronary artery atherosclerosis, and brown fat may be associated with fewer cardiovascular events [311]. The etiology for this protective effect is uncertain [311].
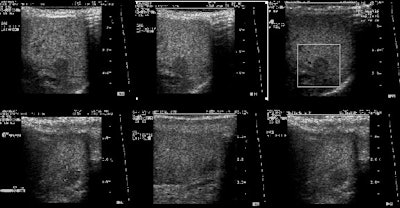
A hibernoma is a benign tumor of brown fat that can show elevated
FDG accumulation on PET imaging [181]. The entity is rare and
typically presents as a slow-growing painless mass in a
subcutaneous or intramuscular location [315]. The most common
location is the trunk, neck, and proximal extremity- particularly
the thigh [315]. Hibernomas appear as a nonspecific echogenic mass
on US and appear slightly denser than subcutaneous fat with clear
margins on CT [315]. Similar to brown fat, the tumor appears to
demonstrate fluctuation in the amount of tracer uptake when imaged
on different dates and are more commonly seen in winter months
[181,315].
|
Brown Fat: Prominent, symmetric supraclavicular tracer uptake was seen in this patient without a corresponding CT finding. Uptake in this area has been felt to be related to the presence of "brown" fat. |
|
|
There is normal tracer uptake in the skeletal growth centers- particularly long bone physes [125]. Uptake in benign bone lesions can be seen, such as Pagets disease and fibrous dysplasia can be seen during their active phases [39,176]. Benign fibro-osseous lesions such as non-ossifying fibromas, fibrous cortical defects, and cortical desmoids can also demonstrate tracer uptake [149,150]. Acute (fractures less than 3 months old [111]) or actively healing fractures also show increased FDG activity [39,92]. Sacral insufficiency fractures can also demonstrate increased tracer activity and may have a linear appearance [66]. Patients with osteoarthritis or bursitis of the shoulders can show diffuse uptake about the shoulder joint [124]. Decreased skeletal activity can be seen following radiation therapy [39].
FDG uptake around the head and neck of hip prosthesis can commonly be seen in non-infected prostheses [49] (whereas increased tracer uptake along the interface between the bone and the prosthesis is suggestive of infection [50]). The artifact is related to differences in density at the interface between the metal prosthesis (high density) and the surrounding soft tissues (low density) [51]. Because of partial volume effects, the scanner records information along this interface as an average of the two densities [51]. This leads to over-correction of the low density soft-tissues adjacent to the prosthesis [51]. This finding can be caused by motion (even of only a few millimeters) between the emission and transmission scans [51]. The use of attenuation-weighted iterative image reconstruction or software based metal artifact reduction on the CT data can minimize this finding [51,299]. Also, this artifact should not be present on non-attenuation corrected images [51].
|
Peri-prosthetic activity: The patient below had a left hip prosthesis. The PET scan was performed for the evaluation of a lung nodule. The patient had no symptoms referable to the left hip. Note the increased activity along the margins of the neck and head portions of the prosthesis. This finding can be seen normally and should not be considered evidence of infection. |
|
|
|
Peri-prosthetic activity: The patient below had bilateral hip prostheses. Note how the periprosthetic tracer uptake (black arrows) becomes less conspicuous on the non-attenuation corrected images (right). |
|
|
Similar to other nuclear medicine examinations, an intra-arterial tracer injection will result in a "glove phenomenon" with significant activity in limb distal to the arterial injection site.
|
Arterial injection: The patient below had an accidental arterial tracer injection. Note the significant increased activity in the left arm below the site of the injection. |
|
|
6- Gastrointestinal tract- variable activity can be seen in the
GI tract- partly due to smooth muscle activity associated with
peristalsis, bacterial uptake, gastrointestinal lymphoid tissue,
and metabolically active mucosa [20,30,54,95].
Small
bowel activity is usually heterogeneous and of low intensity
[156]. Inflammatory conditions such as Crohn's disease can produce
areas of increased tracer activity [156].
Metformin:
Metformin is a biguanide drug used in the treatment of type II
diabetes (by decreasing glucose transport from food to plasma,
decreasing glucose output form the liver and increasing glusoce
uptake in peripheral tissues and enhanced glucose consumption by
enterocytes), but which is also being recognized for having
antineoplastic actions [270,271]. Metformin directly and
indirectly activates adenosine monophosphate-activated protein
kinase (AMPK) through the inhibition of mitochondrial respiratory
complex I [270]. AMPK activation results in the inhibition of
energy-consuming processes (such as DNA synthesis, protein
synthesis, and lipid synthesis) [270]. However, AMPK activation
results in the upregulation of adenosine triphosphate-producing
processes (such as glucose uptake from the circulation,
glycolysis, and fatty acid oxidation) [270]. Hence, metformin can
produce a dose-dependent increase in tumor glucose uptake, while
decreasing cell proliferation [270]. In animal models and tumor
cell lines, changes in FDG uptake following metformin treatment
may be misleading [270].
Increased tracer uptake within the bowel (particularly in
the colon where the uptake is typically intense, diffuse and
continuous) has been reported in diabetic patients taking
metformin [233,271]. The effect appears after a relatively long
period of treatment [271]. The exact reasons for the increased
bowel activity is unclear, but metformin has been found to enhance
the transfer of glucose from the vascular compartment into the
intestinal mucosal cells and increase glucose utilization [318].
The tracer uptake is in the bowel wall and not in the bowel lumen
and hence cannot be cleared with a bowel prep [271]. Stopping
metformin for 2 to 3 days prior to imaging can result in clearance
of the intense bowel uptake [234,236,330]. Stopping the medication
for only 1 day does result in decreased activity compared to not
stopping the medication, but is not as effective as 48 hour
discontinuance [236,330]. A drawback is that longer periods of
discontinuance of the medication can result in a few patients
experiencing elevated glucose levels [330]. Metformin 48 hour
withdrawal may aid in evaluation of patients with abdominal
malignancies, especially when disease is suspected in or in close
proximity to the bowel [330].
|
Metformin bowel activity: A large
amount of bowel activity can be seen in this patient
that was taking metformin. |
|
|
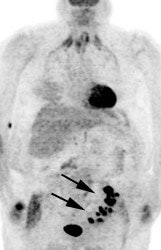
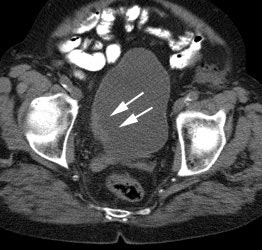
Large bowel activity is common and can be focal, segmental, or diffuse [40]. Focal intense uptake in the colon is an uncommon finding (1-2% of cases) and should be further evaluated with colonoscopy to exclude a neoplastic process which can be found in a large percentage of patients (61 to 86% of focal abnormalities are found to be premalignant or malignant) [40,93,96,114,123,2227]. The visualization rate of colorectal polyps increases with polyp size and the severity of histologic dysplasia [197]. Acute diverticulitis is an inflammatory condition that can result in focal colonic uptake [96]. Segmental colonic uptake is usually related to inflammation, while diffuse uptake is usually not associated with underlying bowel abnormality [40]. Areas of active Crohn's disease will also demonstrate FDG accumulation [170]. Uptake in the cecum and right colon is usually higher than in other colonic segments [4,40]. This may be related to abundant lymphoid tissue in this region [54]. FDG accumulation can also often occur in segments or large sections of the colon after colonoscopy- possibly due to a non-specific inflamation [20]. A perirectal area of artifactually increased curvilinear tracer uptake has been described and has been felt to be related to movement of gas within the rectum [230]. An air pocket present in the rectum during the PET acquisition where there was no gas during the CT exam results in overcorrection for attenuation at the margin of the rectum [230]. Adjacent extremely high tracer concentration in the urinary bladder is felt to contribute to the artifact [230].
|
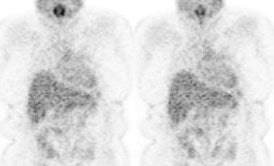
Normal bowel activity: Faint
heterogeneous bowel uptake is common. |
|
|
|
Intense right colon activity: Note the intense activity in the right colon in this asymptomatic patient. Increased activity in the right colon can sometimes be seen. Note uptake in the patients left upper lobe lung cancer. Focal activity in the left upper abdomen was related to the stomach. |
|
|
|
Diffuse large bowel activity: Diffuse increased colonic FDG uptake was seen in this asymptomatic patient. Diffuse uptake is usually not associated with underlying bowel abnormality. |
|
|
|
Focal colonic uptake associated with colon cancer: This patient had focal tracer uptake in the region of the cecum. CT demonstrated a soft tissue mass which was found to be a primary colon cancer on colonoscopy. Focal areas of colonic tracer uptake should be further evaluated. |
|
|
|
Segmental colon activity in diverticular disease: Note the segmental FDG uptake corresponding to an area of severe diverticular disease on CT. There is likely underlying inflammation or muscular spasm which contributes to the FDG uptake.. |
|
|
The stomach is usually faintly seen, but uptake can be intense - activity is seen only in the wall of the stomach (possibly related to smooth muscle activity) giving it a "ring-like" appearance [4,99,111]. However, if the stomach is not distended, uptake can appear focal- particularly in patients with prior partial gastric resection surgery [116]. Drinking a glass of water (some authors recommend 250 mL [228]) will produce gastric distention and benign uptake will generally become less conspicuous [116]. Focal gastric uptake should prompt further evaluation to exclude an underlying mass [156].
|
Gastric activity: Gastric activity can be very intense- even in patients without gastrointestinal disease. Note the "ring-like" appearance of normal gastric activity. |
|
|
There can be normal mild FDG activity in the esophagus possibly due to swallowed saliva or smooth muscle activity and this can potentially obscure subtle lesions [11]. If the uptake in the distal esophagus is linear, but moderate to intense in activity- further evaluation should be performed to exclude Barrett esophagus [156]. There is usually a focus of moderate FDG uptake at the gastroesophageal junction- most likely related to normal contraction of the lower esophageal sphincter (which prevents reflux) [111]. Esophagitis in the distal esophagus is also a common cause of tracer accumulation [4]. However, even in patients without a specific history of esophagogastric disease, the gastric or gastroesophageal junction SUV can be as high as 4.0 (values higher than this should prompt further evaluation)[99]. None-the-less, uptake within the distal esophagus can be associated with underlying malignancy in 2-8% of patients [184]. Findings that are more suggestive of malignancy include focality (rather than linear), eccentric location, and more intense uptake [184].
|
Esophageal activity: The image below demonstrates non-specific esophageal activity which appears as linear uptake anterior to the spine (black arrows). |
|
|
|
Ostomy sites: Tracer uptake at ostomy
sites is common. |
|
|
For examinations that are co-registered with CT imaging, oral contrast can aid in confirming activity is located within bowel [30]. Oral contrast administration is associated with some increased FDG uptake in the ascending colon [30]. Also- because the contrast agent is traveling through the bowel the CT data may not exactly represent the distribution of contrast at the time of the FDG exam [30]. In principle, a mismatch of low CT density at the time of CT data acquisition and high CT contrast density at the time of FDG imaging could result in an artificially reduced FDG activity on attenuation corrected images [30].
7- Thymus: Thymic uptake is commonly seen in children (up to 73% of children prior to chemotherapy) with an SUVmax of 2.00 +/- 0.83 [98,257]. However, not all children will have visible thymic activity on FDG imaging [19]. The cause of this variability in pediatric thymic accumulation of FDG is unclear, but is likely related to physical and emotional stressors which influence thymic metabolism [19]. In general, physiologic uptake of FDG in the thymus disappears in adolescence in conjunction with involution of the thymus [19]. However, physiologic uptake in the thymus can be seen in patients well beyond puberty [98] and thymic FDG accumulation can also be seen in adult patients with a normal thymus [48].
The thymus responds to systemic stress (infection, neoplasm,
chemotherapy, surgery) by rapid atrophy [257]. Once the stressful
event has ended, the thymus may regrow beyond it's original size
[257]. Thymic hyperplasia is one etiology for physiologic FDG
accumulation in the thymus [98]. Thymic hyperplasia represents an
immunologic rebound phenomenon following chemotherapy which is
especially seen in young patients treated for malignancy [98].
Following chemotherapy, thymic FDG uptake can be seen in 75-80% of
children and in 5% to 16% of adults [4,7,8,19,98,203].
Thymic enlargement can persist for up to 6 months (sometimes
longer [257]) following completion of chemotherapy [7].
Occasionally, a lip of thymic tissue can extend above the left
brachiocephalic vein- uptake in this tissue should not be confused
for adenopathy [169]. [257]
There are several clues to non-pathologic thymic FDG accumulation. Normal thymic activity is usually triangular or "V" shaped (bilobed) and generally not very intense [98]- uptake values are typically less than 2.5 [48]. An SUVmax of greater than 3.4-4.0 is felt by some authors to be concerning for malignancy [257]. However, one study indicated that uptake associated with thymic hyperplasia demonstrated SUV max values of 3.73 =/- 1.22 [203]. On anatomic imaging studies, the thymus should also have a normal, homogeneous appearance [98]. Lack of uptake on the pre-therapy scan should also be a clue to post-treatment thymic hyperplasia [98].
FDG accumulation in the thymus suggests pathology when it does not have a typical triangular shape or if the activity is very intense (SUV max greater than 4.0 [98]) [19].
8- Bone marrow- faint activity is generally identified within the bone marrow [7]. The accumulation is generally homogeneous and has SUV ratios between 0.7 to 1.3 [7]. Bone marrow activity that is greater in intensity than the liver is considered abnormal [39]. Increased bone marrow activity can be seen with bone marrow recovery following chemotherapy, but this usually resolves by one month post-therapy [4]. Treatment with granulocyte stimulating factors (Filgrastim [Neupogen- daily dose] and Pegfilgrastim [Neulasta- single dose]) can also produce diffuse skeletal FDG accumulation [9,142] which can interfere with accurate PET imaging by decreasing the bioavailability of FDG to the tumor [79,142,167]. Both agents also result in increased splenic FDG accumulation [142]. Elevated marrow activity may persist in some patients for up to 20 days in some patients [79]. Both agents also result in increase splenic FDG accumulation [142]. Separating PET imaging from short acting colony stimulating factor therapy by a minimum of 5 days is recommended [79] and up to 30 days may be required for long acting agents [147].
Radiation treatment can also affect marrow activity. In an animal model following XRT, the irradiated bone marrow shows a significant increase in FDG accumulation over baseline on day 1, a significant decrease on day 9, and a return to normal between 18 and 30 days [12,13].
|
Marrow activity: The images below are from two separate patients each showing mild FDG accumulation within the vertebral bodies. |
|
|
|
Growth colony effect: The patient shown below had received growth colony stimulating factor (GCSF). Note the extensive increased marrow activity. Note increased splenic activity also seen as a result of GCSF therapy. Click image to view rotating avi file. |
|
|
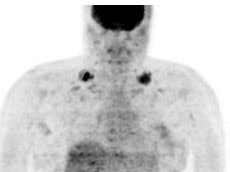
9- Thyroid- Normally, FDG uptake in the thyroid gland is low or
absent as fatty acids are the primary metabolic substrate [166].
Incidental diffuse thyroid uptake can be seen in 0.6 to 3.3% of
patients [166]. Diffuse uptake occurs in association with chronic
lymphocytic thyroiditis (Hashimoto's) [166] or Graves' disease and
is generally felt to be a benign finding [140]. It has also been
suggested that diffuse increased tracer uptake is associated with
decreased attenuation of the thyroid on CT- possibly related to
inflammatory cellular infiltration of the thyroid gland (normally
the thyroid is of higher attenuation due to the presence of iodine
within the gland) [225].
Incidental focal uptake of FDG in the thyroid can be seen in about 2% to 4.3% of scans and is associated with autonomously functioning thyroid nodules and thyroid malignancies [31,120,140]. Patients with focal uptake should be further evaluated due to a higher risk of the finding being associated with thyroid malignancy (21-57% risk for malignancy and most commonly papillary carcinoma) [31,112,120,140,166,185,335]. However, the maximum SUV of thyroid cancer is not statistically higher than that of benign lesions [185]. Benign lesions associated with uptake include degenerate nodules, follicular adenomas, and adenomatous hyperplasia [327]. Oncocytic/Hurthle cell lesions demonstrate intense FDG uptake and this is related to an intrinsic mitochondrial defect driving inefficient glycolytic metabolism [327].
In patients with focal thyroid uptake, correlation with targeted
thyroid ultrasound can provide additional information as nodules
with benign sonographic features are much less likely to be
malignant on biopsy [185]. Other authors recommend that a decision
to pursue further evaluation of focal thyroid uptake should also
be based on the patient's overall prognosis- which may be poor due
to their underlying primary malignancy [327].
|
Normal thyroid activity: Symmetric thyroid tracer uptake can be seen is some patients. |
|
|
10- Lymph nodes- nodal uptake can occur if the agent extravasates into the soft tissues at the site of injection (always inject in arm opposite primary lesion) [4]. Lymphoid tissue can also demonstrate significant uptake- particularly the tonsils and adenoids [20]. Nodal uptake is also seen in patients with sarcoidosis- probably related to activated macrophages within granulomas [80,81]. In sarcoid, the degree of uptake has been related to disease activity and to document response to therapy [80,81].
11- Gonads/Ovary/Uterus/Endometrium-
Male gonadal activity can be seen and is quite variable. The
normal prostate gland usually demonstrates homogeneously low level
FDG uptake [324]. Incidental uptake in the prostate can be seen in
about 1.2-2% of male patients without known prostate cancer and
occult prostate cancer can be found in 5-23% of cases [261,314].
The likelihood of cancer in incidental focal prostate uptake has
been shown to be related the patients PSA level (incidence of
cancer was about 4% with PSA of less than 2.5 and almost 60% with
a PSA greater than or equal to 2.5 [314]. Other authors suggest
that incidental focal prostate uptake can be found in 1.8% of FDG
PET exams and has a significant risk for malignancy of 62$ [324].
Faint uterine (endometrial) activity is common. In premenopausal patients, greater endometrial accumulation can be seen in the uterus during menstruation and ovulation (mid-cycle)- typically days 0-4 and approximately day 14, respectively [20,77,318]. Uptake is lower during the proliferative and secretory phases [77]. This is because the first half of the uterine cycle is characterized by increased glycolysis under the stimulation of estrogen and glycogen synthetase, while the second half is characterized by breakdown of glucose under glycogen phosphorylase activity [129]. Mean endometrial SUV in premenopausal women has been reported to be 5+/- 3.2 during menstruation, and 3.7 +/- 0.9 during the ovulatory phase [227].
Only mild endometrial activity may be seen in post-menopausal women (mean endometrial SUV is about 1.7 +/- 0.5) and does not seem to be significantly affected by hormone or anti-breast cancer hormone therapy [77]. Therefore, increased endometrial FDG uptake in postmenopausal women should be viewed as suspicious and may indicate malignancy [227,318].
Uterine fibroids usually show mild FDG uptake, however, they are also known to occasionally show increased FDG activity (up to 18%) [176,227]. There can even be variable heterogeneous uptake within the same fibroid [318]. Fibroids with increased FDG uptake are more common in premenopausal than postmenopausal women [227]. In premenopausal women, fibroids tend to show higher uptake during the lureal phase than during the menstrual, follicular, and preiovulatory phases [227].
Adenomyosis generally shows mild FDG uptake in premenopausal women and the uptake is often higher during the menstruating and ovulating phases [227].
Increased ovarian uptake can be normal in premenopausal patients and is associated with ovulation and corpus luteal cysts- and is postulated to be due to an inflammatory reaction involving cytokines during the ovulatory process to increase glucose uptake to meet the metabolic demands of the growing follicle [77,214,218]. Ovarian uptake in post-menopausal women is associated with malignancy and should always prompt further evaluation [77]. Symmetric uptake in the fallopian tubes has also been described in premenopausal women at mid-menstrual cycle [223].
|
Normal uterine activity: Faint uterine uptake is common (black arrows). |
|
|
|
Normal testicular activity: Mild symmetric testicular uptake can be seen (black arrows). |
|
|
12- Degenerative joint disease and degenerative disk disease can be associated with increased tracer accumulation [146]. Incidental uptake suggestive of degenerative changes in the spine can be seen in up to 22% of patients [146]. The lumbosacral spine is the most common location and the severity of uptake correlates with the severity of the degenerative changes noted on CT [146]. The FDG accumulation is likely related to the inflammatory process that accompanies degenerative joint disease [146]. PET/CT aids in proper localization of the uptake so that it is not mistaken for metastatic disease [146].
13- Vascular activity- in scans not corrected for transmission,
vascular activity in the large vessels in the thighs and pelvis
can be seen in about 80% of patients [10]. On attenuation
corrected images mild vascular activity can be seen in association
with severe atherosclerotic disease or aneurysms [39]. Vascular
uptake likely reflects active atherosclerosis [68,186,248] and is
probably related to the presence of macrophages within the plaque
[89,97,119,199,202]. FDG vascular
uptake is rarely seen in areas of vessel calcification [248]. High
carotid FDG uptake is commonly associated with increased clinical
risk factors and the presence of vessels with 18F-FDG
uptake has also been associated with greater atherogenic
risk/acute plaque events such as stroke or MI [186,248,250,285].
This is because vulnerable plaques have a large, hypoxic,
metabolically active core containing lipid, oxidized lipid, and
inflammatory cells (predominantly macrophages) [199]. Vulnerable
lesions have a thin fibrous cap that can be weakened by the
secretion of proteolytic enzymes from the inflammatory cells
[199]. Atherogenic uptake can be decreased with life style
modifcation and the magnitude of decreased activity appears to
correlate with increased plasma HDL [186]. Vascular uptake is also
seen in association with thrombophlebitis (which can be very
intense) [39], vasculitis [97,115], and has been described in
pulmonary embolism [171].
Vascular grafts also demonstrate mild tracer accumulation, as do
sites of endarterectomy [39]. In one study, high FDG accumulation
was identified in 75% of non-infected aortic vascular grafts
placed by open surgery (and in one of 4 grafts placed
endovascularly) [189]. In another study, diffuse FDG uptake was
found in 92% of non-infected vascular prostheses and uptake was
seen for up to 16 years with no change in intensity [286]. Uptake
in vascular grafts is likely the result of a sterile foreign-body
inflammatory reaction [286].
Injections performed via a port-a-cath will show retained activity in the hub and also along the course of the catheter [39].
|
Normal vascular activity: Faint activity can be seen in the descending thoracic aorta (black arrows) |
|
|
|
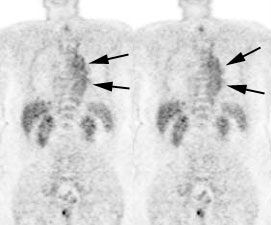
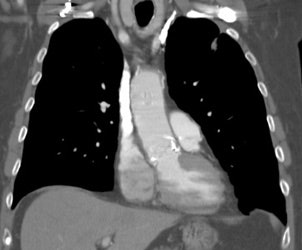
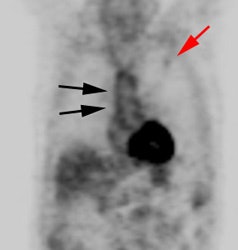
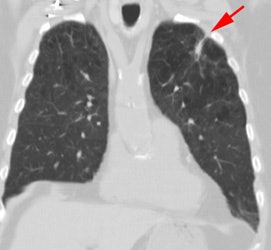
Vascular graft activity: The patient below had a prior graft repair of an ascending aortic aneurysm. Tracer uptake can be seen in the ascending aortic graft (black arrows). The patient also has a left upper lobe lung cancer (red arrows). |
|
|
|
Vascular graft activity: Another example of an asymptomatic patient with an aortic stent graft demonstrating tracer accumulation on FDG PET imaging. There is also inflammatory tracer uptake about the hips and left shoulder. |
|
|
14- Breasts- Incidental foci of tracer
uptake in the breast can be seen in 0.36%to 1.1% of PET scans
[247]. Any focal area of increased tracer accumulation in the
breast should prompt further evaluation to exclude an underlying
malignancy [144,334] (incidental focal breast uptake is associated
with malignancy in 37.5 to 57% of patients (however, a malignancy
rate as high as 83% has been reported) [247]). Variable
uptake can be seen within glandular tissue [20,61].
Higher FDG uptake is seen in women with dense breasts (larger
amount of glandular tissue) and in women on hormone replacement
[61]. The maximum peak SUV observed in dense breasts can be as
high as 1.39 [61] (in another study the average SUVmax of dense
breasts was 1.24 [215]). The average SUV is typically 0.50 to 0.69
[61,215]. The nipples also normally demonstrate FDG activity-
especially on non-attenuation corrected images [111]. High uptake
of FDG can be seen in the lactating breast due to intracellular
trapping within active glandular tissue [21].
There is low excretion of FDG into breast milk and the estimated
cumulative dose to a breast fed infant is approximately 0.085 mSv
(well below the 1 mSv recommended for cessation of breast-feeding)
[21,276]. The ICRP does not recommend interruption of breast
feeding after FDG administration [276]. Although the amount of
radioactivity within breast milk is low, the retained activity
within the breast poses a direct and significant source of
radiation exposure to the nursing infant [21].Therefore, close
contact with the infant should be avoided for 12 hours following
tracer injection [21,276]. It is recommended that the infant be
breastfed just before injection, to maximize the time between
injection and the next feeding [276]. Milk pumped from the breast
may also be fed to the infant via a bottle [276].
|
Normal breast activity: Faint activity can normally be seen in the breasts (black arrows) |
|
|
15- Spleen- Diffusely increased splenic FDG uptake can be seen in association with granulocyte colony-stimulating factor treatment [32,33].
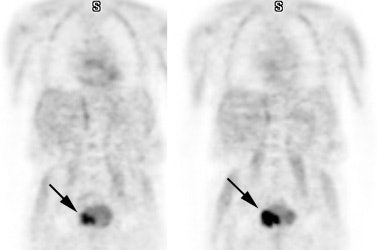
16- Benign adrenal lesions: Adrenal masses are not rare in the general population (2-9%), however, the adrenal is also a common site of metastases in cancer patients [190]. In general, lack of FDG uptake in an adrenal lesion is highly predictive of a benign etiology (NPV 93%). Unfortunately, benign adrenal adenomas and rarely adrenal myelolipomas (hematopoietic elements) can have uptake of FDG equal to or greater than the liver resulting in a false positive exam in (between 5% to 15% of adrenal adenomas/benign adrenal nodules demonstrate false positive FDG uptake) [135,137,155,165,176,190]. False negative exams can also occur with neuroendocrine metastases, small lesions, necrotic/hemorrhagic lesions, and in patients that have received previous therapy [135,155,190]. Generally, however, adenomas demonstrate only mild tracer accumulation that is less than liver activity, but greater than background [135], whereas malignant lesions will show uptake that is greater than liver activity [155].
The use of PET/CT can improve characterization of adrenal masses by improving the exams specificity [136,155]. Using activity equal to or greater than the liver to indicate malignancy, PET/CT imaging has a sensitivity of 83-100%, a specificity of 85-97%, a PPV of 67-87%, and an NPV of 93-100% for the characterization of adrenal lesions [190,198]. A cutoff SUV of 3.1 has been suggested to have a sensitivity of 98.5% and a specificity of 92% for characterization of malignant adrenal lesions [136]. However, between 3-10% of benign adrenal lesions will demonstrate increased FDG accumulation [198]. PET/CT permits further characterization of the lesion with an HU measurement of less than 10 indicative of a benign adrenal lesion [136,137,155].
|
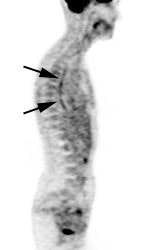
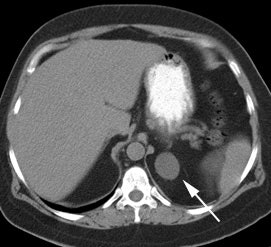
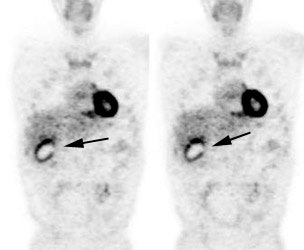
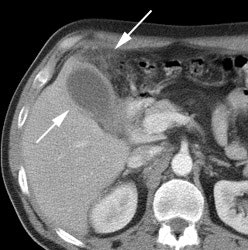
Benign adrenal lesion with FDG uptake: The patient shown below had a left adrenal mass which had remained stable for 2 years (white arrow). The stability over time and CT features would be consistent with a benign adrenal lesion- likely an adenoma. Note the marked FDG uptake within the mass on PET imaging (black arrows). Benign adrenal adenomas can accumulate FDG resulting in false positive exams. The use of PET/CT has been shown to improve the specificity of the PET exam. |
|
|
17- Inflammation- FDG accumulation can be seen at sites of inflammation. The increased accumulation is probably related to a markedly increased rate of glycolysis within activated inflammatory cells. Talc pleurodesis produces a visceral and parietal pleural granulomatous inflammation. Increased FDG activity has been reported in sites of pleural talc (up to 3 years following the procedure)- presumably secondary to pleural inflammation [71,72]. Diffuse increased FDG accumulation in the lungs can be seen in association with bleomycin pulmonary toxicity [104]. Pulmonary accumulation of FDG has also been described in patients with idiopathic pulmonary fibrosis - particularly in regions of reticulation/honeycombing [201].
Mesh used for hernia repairs can also be associated with FDG accumulation and can be seen for several years (SUV max 2.8 to 5.6) [108]. The uptake is likely associated with a foreign body granulomatous reaction and it seems to be more intense with polypropylene mesh compared to polytetrafluoroethylene mesh [108].
|
Inflammation: The image below demonstrates ring-like increased tracer activity surrounding the gallbladder in a patient with cholecysitis (black arrows). Note the gallbladder wall thickening and adjacent inflammatory changes seen on the patient's CT scan (white arrows). |
|
|
18- Hilar uptake- bilateral
hilar uptake of FDG has been reported in patients without lung
cancer [187]. Findings that suggest a benign etiology include
symmetric, low level (SUV max < 3) hilar uptake [187].
Asymmetric uptake and the presence of uptake in other
mediastinal nodes should raise the degree of suspicion for
malignancy [187].
Other agents used for oncologic imaging:
18F-Fluoride:
18F-fluoride (18F-NaF) is a bone imaging
agent used for PET [78]. 18F-fluoride is produced by
11-MeV proton irradiation of 18O-water in a tantalum
target body using a cyclotron [237]. The irradiated aqueous
solution containing 18F-fluoride is diluted with
sterile water and passed through cation and anion exchange
cartridges to trap the 18F-fluoride [237].
18F-NaF is an analog of the hydroxyl group in
hydroxapatite bone crystals (fluoride ions replace the hydroxyl
ions in hydroxyapatite) [324,336]. After diffusing into the
extracellular fluid of bone, the fluoride ion is exchanged for a
hydroxyl group on the hydroxyapatite crystal bone and forms
fluoroapatite which then incorporated into the bone matrix on the
bone surface [78,122,180,324]. Tracer uptake correlates with bone
remodeling and osteoblastic activity, as well as blood flow [324].
18F-NaF has a biodistribution similar to Tc-99m-MDP,
but it's lower protein binding in the blood (about 30% of the
Tc-MDP is protein bound immediately after injection) allows a more
rapid single pass extraction by bone (nearly 100% first-pass
extraction by bone [336]) and fast clearance from the soft tissues
(thus permitting earlier imaging- as early as one hour following
injection) [249,289,294,336].
Ion exchange is the mechanism of uptake and blood flow is the
rate limiting step in the transfer of fluoride ions from the blood
to bone [313]. About 50% of the injected dose localizes to
bone and essentially, all the 18F-fluoride that is
delivered to bone is retained in the bone [177]. 18F ions exchange with hydroxyl ions
(OH-) on the surface of the hydroxyapatite to form
fluoroapatite [294]. However, as the actual process of
binding to bone takes hours to days, during one hour post
injection imaging the agent is unlikely to have been incorporated
into bone, but rather it is likely bound within the bone's
extracellular fluid [263]. Bone uptake of the fluoride ion is
two-fold higher than that of 99mTc-polyphosphonates
and because it is not protein bound there is faster blood
clearance (resulting in a better target-to-background ratio)
[78,122,219,249]. Tracer uptake is higher in new bone because of
higher availability of binding sites [232]. The concentration of 18F-NaF is 10 times higher in area s of
regenerating bone, compared to areas of normal bone [294]. Local
or regional hyperemia may also cause increased tracer localization
in the skeleton [232]. Processes that result in minimal
osteoblastic activity, or primaily osteolytic activity, may not be
detected [232].
Following IV administration, the agent is rapidly cleared from the plasma in a biexponential manner [177] and excreted by the kidneys [237]. The first phase has a half-life of 0.4 hours and the second phase has a half-life of 2.6 hours [177]. The rate limiting step of 18F-NaF uptake is blood flow as almost all of the agent is retained in bone after a single pass [237,261]. One hour after administration, only about 10% of the injected dose remains in the blood [177,313]. There is negligible plasma protein binding of the agent [237,289]. Fluoride ions are freely filtered in the glomeruli, but fluoride also undergoes tubular resorption and the tubular absorption increases with decreasing GFR [275].
The rapid uptake and clearance allow earlier imaging (typically within one hour [as early as 30-45 minutes following administration]) with an improved target-to-background ratio and better image quality compared to Tc-MDP [177,219,249]. Another benefit of PET compared to gamma camera imaging is the improved spatial resolution (4-5mm) compared to 10-15 mm for Tc-MDP imaging [219,249]. On average, 15 +/- 5% of the whole body activity is excreted into the urinary bladder [263]. Urinary excretion of the agent can result in image artifacts that may obscure lesions in the sacrum or lower pelvis- hence patients should void prior to imaging [177]. The spatial resolution of 18F-fluoride is also superior to standard bone scanning [78]. 18F-fluoride imaging has been shown to be more sensitive and specific in detecting skeletal metastases than planar or SPECT MDP bone imaging [177,288,289]. In a meta-analysis comparing bone scan with 18F-fluoride PET, the pooled sensitivity and specificity for 18F-fluoride were 96 and 98%, respectively, compared to 57% and 98%, respectively for bone scan [288]. In the National Oncologic PET registry study, NaF PET scan results were associated with a change in management in 40% of patients - particularly prostate and breast cancer patients [297]. However, another study suggested that although 18F-fluoride PET/CT can result in a significant reduction in equivocal readings compared to planar bone scan, it may not have a significant impact on changing tumor staging [325].
18F-Fluoride is very sensitive for the detection of both lytic and sclerotic bone lesions, however, the agent is not tumor specific and benign bone lesions (degenerative change, fractures, Pagets disease, enchondroma, and osteoma) will also demonstrate increased tracer uptake [78]. 18F-fluoride is more likely to detect skeletal metastases from tumors that typically have low FDG activity [177]. FDG PET is more likely to detect bone marrow metastases or small osteolytic lesions (lesions with little or no increase in cortical turnover) [177]. Improved specificity is seen when the 18F-fluoride scan is interpreted with a fused CT exam [78,133]. Lesions that measure less than 3 mm have limited detectability on 18F-fluoride PET [177]. A combined 18F-fluoride and 18F-FDG scan protocol has been proposed to maximize the strengths of each agent [200]. A "flare" phenomenon can be seen in patients with breast cancer following initiation of therapy [177]. 3-phase imaging is not presently possible [177].
Patients should be well hydrated for the exam to promote rapid renal excretion of the agent- decreasing radiation dose and improving image quality [232]. Unless contraindicated, patients should drink 2 or more 8-oz glasses of water within 1 hour before the exam, and another 2 or more glasses of water after tracer administration [232]. No fasting is necessary and patients may receive their usual medication [294]. The bladder should be emptied immediately prior to imaging [232]. Scanning is performed 60 +/- 30 minutes minutes after the injection of 8-12 mCi (2.1 MBq/kg) of 18F-fluoride [78,177,262]. The SNM practice guidelines indicate that the adult dose should be 185-370 MBq (5-10 mCi) with the higher dose being used in obese patients [232]. Pediatric activity should be weight based (2.22 MBq/kg or 0.06 mCi/kg) using a range of 18.5-185 MBq (0.5-5 mCi - the pediatric dose should not exceed 5 mCi) [232,294]. To obtain high quality images of the extremities, a longer delay (90-120 minutes) may be required [232,263]. The total imaging time is 15-30 minutes- therefore an entire exam can be completed in approximately one hour [177]. The SNM guidelines indicate that in patients with normal BMI, good images of the axial skeleton can be obtained with an acquisition time of 3 min/bed position starting 45 minutes after the injection of 5 mCi of 18F [232]. Good whole body imaging can be obtained with using 3 min/bed position starting 2 hours after injection of 10 mCi of 18F [232].
Radiation dosimetry: There is little overall difference in the
estimated radiation dose for 18F-fluoride and Tc-MDP
scintigraphy [177], however, other authors indicate that the dose
from a 18F-NaF bone scan is higher
than that from a Tc-MDP bone scan [294]. Although the
radiation absorbed dose from the agent is higher, a lower dose can
be used and the actual absorbed dose is similar to that from MDP
bone scanning [249]. 18F-fluoride delivers radiation
from both the emitted positron (mean energy 250 keV) and the two
511 keV annihilation protons [177]. The proton will deposit
essentially all of its kinetic energy in the source organ, while
the 511 keV photons have a soft-tissue half value layer of 7.3 cm-
meaning energy will be delivered to, as well as distant from, the
source organ (140 keV photons of Tc-99m deposit most of their
energy in the source organ) [177]. However, 18F has a
shorter half-life than Tc99m (110 minutes versus 6 hours) so there
is a shorter period of exposure [177]. For a 70 kg patient the
effective dose from a 18F-fluoride
exam is about 4.0 mSv (compared to 3.0 mSv from a Tc-MDP exam)
[177], although other authors report the dose from a 10 mCi scan
to be 8.9 mSv (compared to 5.3 mSv from a 25 mCi Tc-MDP scan)
[275,294,324]. An additional 1.0-4.0 mSv dose is incurred if CT is
used for attenuation correction and anatomic localization
[289,294]. The urinary bladder receives the highest dose, followed
by the bone surfaces and red marrow (compared to Tc-MDP in which
the bone surface receives the highest radiation dose)
[262,275,289,294].
In the National Oncology PET registry trial, 18F-fluoride PET/CT changed management from non-treatment to treatment in 47% of patients at initial staging, 44% of patients with suspected first bone metastasis, and 52% of patients with suspected progressive disease [336].
Both benign and malignant bone lesions will demonstrate 18F-NaF uptake, including fibrous dysplasia and vertebral hemagiomas [294,309]. Extraosseous uptake of 18F-NaF can be seen in the arterial vasculature (likely related to atherosclerotic calcification), gastrointestinal tract, and genitourinary tract (due to urinary bexcretion) [294]. Uptake can be seen in large and small bowel and the etiology is uncertain [294]. Similar to MDP, NaF imaging can be subject to a flare phenomena associated with successful systemic therapy [297].
Combined 18F-Fluoride and 18F-FDG PET/CT scanning: Co-injected combined 18F-Fluoride and 18F-FDG imaging has been studied as an optimum method for the detection of both lytic and blastic bone metastases [267,268,303]. Both agents are injected simultaneously using approximately 10-15 mCi of FDG and 5 mCi of 18F-Fluoride [268,303]. In patients with breast and prostate cancer, combine imaging has been shown to be superior to Tc-MDP scintigraphy and whole body MRI for the evaluation of skeletal disease [303]. However, certain lesions may become less conspicuous on comboned imaging exams [268]. Small lung nodules seen on FDG imaging, may become less evident on combined imaging and metastatic foci in the skull may also not be identified [268]. Another limitation is that, it is not possible to determine if 18F-Fluoride positive/ 18F-FDG negative bone lesions represent viable tumor versus an osteoblastic response to therapy, unless a baseline scan is available for comparison [267,268].
Somatostatin receptor imaging agents:
68Ga-DOTA-Tyr3-Octreotide (68Ga-DOTA-TOC), 68Ga-DOTA-Tyr3-Octreotate (68Ga-DOTA-TATE), 68Ga-DOTA-[1-naI3]-Octreotide (68Ga-DOTANOC):
These agents specifically bind to somatostatin receptors and can be used for imaging neuroendocrine neoplasms which are frequently not well evaluated on FDG imaging due to a low proliferation rate [161,162,216]. Most well-differentiated neuroendocrine tumors express a high density of surface somatostatin receptors that allow imaging with somatostatin receptor scintigraphy [208].
68Ga is conveniently produced by a 68Ge/68Ga
generator [175]. 68Ge with a long half-life
(270.8 days) produces the short-lived 68Ga (T1/2=
67.71 min) [162,175]. 68Ga is an excellent positron
emitter with 89% positron branching accompanied by a low photon
emission 1,077 keV, 3.22%) that decays to stable 68Zn
[175]. Labeling is straightforward and can be performed in a very
short time (under 30 minutes) with good labeling efficiency
[161,162]- although the eluate from the commercial generator still
contains measurable activities of long lived 68Ge
[175].
Prior to performing PET imaging with 68Ga, it is
important to be sure that the PET system is properly calibrated
for the agent (most PET systems are calibrated for 18F),
otherwise, SUV measurements can be underestimated by an average of
15% [328]. 68Ga has a higher energy positron than 18F
(1.9 MeV vs 0.63 MeV) and will travel further prior to an
annihilation reaction [328]. Additionally, 68Ga is
accompanied by a prompt 1.08 MeV gamma emission of approximately
3% abundance which complicates the emission spectrum [328].
68Ge-labeled somatostatin analog peptides clear rapidly from the blood, with reported peak tumor uptake occurring at about 70 minutes and no radioactive metabolites detected in the serum at 4 hours [278].The normal biodistribution of the agent is the spleen, liver, and GU tract due to excretion [161]. Uptake is also seen in the pituitary, adrenal glands, and in the normal pancreatic head (up to 68% of patients) [161]. Faint activity can be seen in the salivary tissue and GI tract [161]. 68Ga-DOTANOC uptake has also been reported in the fibrotic areas of the lungs in patients with interstitial lung disease [239]. The optimal imaging time is 100 minutes following injection [161]. The agent shows a very high affinity for somatostatin receptors 2 and 5 [162]. The agent has been shown to be superior to conventional CT imaging and octreotide SPECT imaging for the evaluation of neuroendocrine tumors with a sensitivity of 97%, specificity of 92%, and an accuracy of 96% on a per patient basis [161]. 68Ga-DOTA-TOC imaging can provide further clinically relevant information in up to 14% of patients compared to SPECT, and up to 21% of patients compared to conventional CT imaging [161]. Uptake does not appear to be influenced by the functional status of the tumor [162].
Interestingly, the agent 68Ga-DOTA-TATE can also demonstrate macrophage active plaque within vessels- even the coronary arteries [212]. This is related to the expression of somatostatin subtype 2 receptors on macrophages and the lack of physiologic cardiac uptake of the agent [212].
Gluc-Lys ([18F]FP)-TOCA:
Gluc-Lys ([18F]FP)-TOCA is an agent that can be used for somatostatin receptor imaging [139]. [111In]DTPA-octreotide imaging is limited by delayed imaging, high physiologic uptake in the liver and kidneys, and the low spatial resolution of SPECT imaging [139]. ([18F]FP)-TOCA has low lipophicity, rapid renal tracer elimination, low liver and intestinal uptake, and rapid tumor uptake (by about 30 minutes) resulting in excellent tumor-to-non-tumor ratios [139]. Imaging should be initiated between 30-40 minutes following tracer injection [139]. Whole body radiation exposure is about 1.3 mSv/100MBq (compared to 8 mSv/100 MBq with [111In]DTPA-octreotide) [139]. In a comparison with [111In]DTPA-octreotide imaging, PET detected over twice as many lesions [139]. The drawbacks of the agent are that it requires a long multi-step preparation and there is limited radiochemical yield (20-30%) [139].
Amino Acids and Other Agents:
Amino acid transport and protein synthesis are generally increased in malignant cells and amino acid imaging is less influenced by inflammation [18,56]. Amino acid transporters mediate transfer of amino acids across cell membranes [280]. Two amino acid transporters, the system L transporter type I (LAT1, SLC7A5) and the system ASC transporter type 2 (ASCT2, SLC1A5) are upregulated in a wide range of human cancers, are associated with a worse prognosis, and are potential targets for tumor therapy [280]. The replacement of a carbon atom by 11C does not chemically change the molecule [18]. Amino acid agents can cross an intact BBB and typically have low CNS uptake which provides good contrast with tumor uptake (compared to FDG which has significant CNS activity) [18,280,290]. The uptake is mediated by increased type L amino acid carriers (facilitated transport is upregulated due to increased transporter expression in tumor vasculature and does not depend on a breakdown in the blood-brain barrier [290]. Images are usually performed within the first hour after tracer administration and patients should be fasting prior to the exam [18]. However, the optimal dietary state for imaging has not yet been clearly defined and tumor uptake of certain amino acids may be promoted by preloading with other amino acids such as tryptophan [34]. Tumor hypoxia decreases uptake of amino acids (unlike FDG uptake which is increased by tumor hypoxia) [26].
18F-fluorodeoxythymidine:
Tumor growth rate is a useful prognostic indicator of tumor
aggressiveness and tumor response to therapy [70]. Cell lines and
tissues with a high proliferation rate require high rates of DNA
synthesis [70]. Thymidine is incorporated into DNA, but not RNA
[279]. PET imaging can be used to measure tumor cell proliferation
using thymidine analogs.
18F-fluorodeoxythymidine (18F-FLT) is an
amino acid agent labeled with 18F that can be used to
measure tumor cell proliferation [35,63].
The agent is transported into the cell by the same nucleoside
carriers as thymidine [84]. The agent is then phosphylated within
the cell by thymidine kinase-1 (TK1) which is
upregulated in rapidly dividing tumor cells especially during the
S-phase of the cell cycle (thymidine kinase activity is a marker
of cellular proliferation and TK1 activity increased
almost 10-fold during DNA synthesis) [35,69,151,274]. There is
prolonged intracellular retention of 18F-fluorodeoxythymidine
because it is polar and cannot cross
the cell membrane and it is also very resistant to catabolism by
thymidine phosphorylase [35]. Only minimal amounts of 18F-FLT
is
incorporated into DNA (0.2% to less than 1%) because the agent
lacks a 3'-hydroxyl group which is essential for chain propagation
[100,102,152, 279]. Imaging is started approximately 60 minutes
following tracer administration [138].
Normal 18F-FLT high uptake can be seen high proliferating tissue such as the bone marrow [279]. High liver activity is seen because FLT anabolism occurs primarily in the liver to produce a glucuronide conjugate that is exported to the blood and cleared by the kidneys (which results in renal and genitourinary activity) [152]. The organs receiving the largest radiation dose from 18F-FLT are the urinary bladder (abour 650 mrad/mCi) and the liver (about 200 mrad/mCi) [65]. The effective dose equivalent is about 100-120 mrad/mCi [65].
18F-FLT has limited ability to image the bone marrow
(because of intense uptake associated with normal cellular
proliferation) and the liver (because of glucuronidation) [64,69]. Minor intestinal uptake can be
observed [69]. Generally, there is no uptake within the brain (the
agent does not cross the blood brain barrier) or myocardium [69,94,279]. Therefore, FLT may be more
favorable than FDG for imaging brain metastases [109]. However,
one must keep in mind that breakdown of the blood brain barrier
can lead to increased activity in the absence of cell
proliferation [279]. Because of the high marrow uptake, FLT
imaging can actually be used to measure health and proliferative
marrow- especially in patients undergoing bone marrow
transplantation [279].
Overall, however, uptake of 18F-FLT within malignant lesions appears to be less than 18F-FDG uptake and there is an increased risk for false negative exams (tumor uptake of FLT, as measured by SUV values, is only about half that of FDG) [63,138,224,279]. Unfortunately, due to it's lower sensitivity compared to FDG, FLT is not useful for staging and restaging lung cancer [94,138] and esophageal cancer [105]. It has also been shown to have low specificity and low positive predictive value in the evaluation of lymph node metastases in patients with head and neck cancers due to non-specific accumulation in reactive nodes [165]. However, 18F-FLT appears to have utility in the imaging of CNS neoplasms due to its low basal CNS uptake which allows better delineation of lesions compared to FDG CNS imaging [117]. See also CNS tumor imaging
Unlike FDG, there is little accumulation of FLT in areas of
inflammation [84]. However, accumulation at inflammatory sites has
been reported and can lead to false positive exams [138].
Inflammatory lung disease can be associated with lymphocytic
infiltrates and involve growth factors that lead to increased
proliferation [138]. FLT accumulation at sites of inflammation
appears to be related to: 1) High proliferation rates of
B-lymphocytes and macrophages; and 2) Nonspecific increased
accumulation due to increased perfusion and vascular permeability
[138,229]. Non-tumoral uptake has also been described in reactive
lymph nodes- likely due to the proliferation of reactive
B-lymphocytes [].
18F-2'-fluoro-5-methyl-l-beta-D-arabinofuranosyluracil (FMAU):
18F-FMAU is a thymidine analog that is phosphorylated
by thymidine kinase and incorporated in DNA [261]. FMAU is
preferentially phosphorlyated by the mitochondrial TK2 in
comparison with cytosolic TK1 [261]. The agent is useful for
imaging tumor proliferation and for imaging reporter gene
expression using herpes simplex virus type 1 TK system [261]. FMAU
is resistant to degradation, undergoes rapid blood clearance, and
has very little accumulation in bone and in the urinary bladder
[261].
18F-fluoro-L-tyrosine:
18F-fluoro-L-tyrosine (18F-TYR) is an agent that can be used to assess the rate of protein synthesis within malignant lesions [56]. The agent is not actually incorporated into proteins, but exhibits high uptake in tumor cells due to increased transport via the amino acid transport system [106,132]. The agent has low uptake in inflammatory conditions thus promising a higher specificity for the detection of malignancy [106,132]. There is high uptake in the pancreas, the salivary glands, and to a lesser extent within the liver which can hamper detection of lesions in these areas [56]. FDG PET imaging is superior to 18F-TYR for staging NSCLC, head and neck tumors, and lymphoma [56,132]. Overall, 18F-TYR has been shown to be inferior to FDG for general tumor evaluation [106], but can be used for CNS tumor evaluation [163]. The agent is also taken up in meningiomas [164].
18F-fluoroacetate:
18F-fluoroacetate may be an alternative to 11C-acetate for prostate cancer imaging [160]. However, fluoroacetate is a toxic compound that occurs naturally- the human median lethal dose (LD50) is approximately 2-10 mg/kg [160]. Fortunately, the amount given in association with 18F PET imaging is 10-6 times BELOW the human LD50 [160].
18F-fluorodihydroxyphenylalanine (DOPA):
18F-FDOPA (an analog of L-DOPA) is an agent being explored for imaging of neuroendocrine tumors (APUD tumors- amine precursor utake and decarboxylation) [87,172]. The agents use is based on the capacity of endocrine tumor cells to take up, decarboxylate (via amino acid decarboxylase- an enzyme that is strongly expressed in neuroendocrine tumors [182]), and store amino acids such as dihydroxyphenylalanine [148]. The agent enters the cell through the amino acid transport systems for large neutral amino acids [182]. For the examination, patients should fast for at least 6 hours prior to the study [148]. The dose is 5MBq/kg body mass. Immediately following injection a set of images over the abdomen is obtained prior to excretion of FDOPA by the biliary tree which is seen on images obtained after 1 hour [148]. Normal tracer uptake can be seen in the striatum and pancreas, and there is elimination via the biliary (resulting in gallbladder visualization), digestive, and urinary tracts (kidneys and urinary bladder are seen) [148,182]. Liver and myocardial uptake are generally faint [173]. Low level uptake can be observed in the small intestine and peripheral muscles [182]. In children, some uptake in the growth plates can be seen [182]. Pancreatic uptake can be nearly completely blocked by the administration of carbidopa [182]. There is minimal uptake in the normal adrenal medulla [182]. The radiation dose is estimated to be 0.02 mSv/MBq [182]. The agent has demonstrated superior performance for lesion identification compared to somatostatin receptor scintigraphy (overall sensitivity for 18F-FDOPA about 85% compared to 58% for SRS) [87]. 18F-FDOPA has been shown to be superior to CT in the detection of skeletal metastases [87]. 18F-FDOPA exam findings can alter patient management in up to 30% of cases [87].
18F-FDOPA has also been shown to be highly accurate for the evaluation of CNS neoplasms [141] due to the excellent visualization of both high-grade and low-grade brain tumors [231]. 18F-FDOPA is brought into tumor cells via amino acid transporters [231]. 18F-FDOPA uptake is higher in high grade compared to low grade lesions [231].
Carbidopa is routinely used for 18F-FDOPA neurologic imaging as it increases striatal uptake, mainly by increasing the agents plasma concentration via decreased renal excretion [182].
18F-17beta-estradiol (FES):
The agent is an analog of estradiol and binds with high affinity
to the antigen receptor in breast cancer [196].
18F-fluciclovine:
The agent is a leucine analog that depicts amino acid transport
into cells [322]. It has been used for the detection of
recurrent prostate cancer [322]. The agent demonstrates rapid
tumor uptake and gradual decrease avidity over time that may be
due to tracer clearance from tumor cells as the agent is not
modified intracellularly [322]. The agent has prominent
physiologic hepatic uptake that can degrade detection of liver
metastases [322]. See also discussion in prostate
cancer section.
18F-fibroblast
activation protein inhibitors (FAPI):
Cancer-associated fibroblasts play a role in the development of
an immunosuppressive tumor microenvironment by creating a dense
desmoplastic stroma that excludes T-cells from tumor deposits
and through secretion of various chemokines that recruit
myeloid-derived suppressor cells and regulatory T cells
[338,341]. Fibroblast activation protein (FAP) has been shown to
be up-regulated in the stroma of more than 90% of epithelial
cancers [340]. FAP expression is associated with increased local
tumor invasion, increased risk of lymph node metastases, and
decreased survival [340]. However, FAP can also be expressed in
benign diseases and in normal tissues during remodeling
including stromal cells and mesenchymal cells during
embryogenesis, wound healing, fibrotic reactions, and
inflammatory conditions such as arthritis, atherosclerotic
plaques, and fibrosis (such as following myocardial infarction)
[340].
FAPI's are peptidomimetic quinoline
derivatives that bind with high affinity to fibroblast
activation protein expressed on cancer-associated fibroblasts
[338]. Hence, these agents will depict the tumor stroma, whereas
FDG imaging shows tumor cell glycolysis [338].
FAPI sensitivity is affected by the amount of
tumor stroma (the supporting tumor stroma is typically
significantly larger than the tumor volume) and the level of FAP
expression [338]. Specificity is limited by FAP expression in
wound healing, active inflammation, fibrosis (such as in the
liver in cirrhosis and in the lung with ILD), and high
fibroblastic activity in normal tissues (such as the
endometrium) [338]. Background organs with low FAPI uptake (but
high FDG uptake) include the brain, liver, digestive system, and
the head and neck mucosa [338].
While changes in FDG uptake reflect viability
of tumor cells after treatment, stromal changes with treatment
are more complex [339]. More information will be required about
modulation of fibroblastic activity, particularly following
radiation, which can induce fibrosis [339].
18F-Fluciclatude:
The agent is used for imaging angiogenesis in
tumors and can be used as a biomarker of response to
antiangiogenic therapies [302]. It contains the RGD sequence
that binds with high affinity to integrins alphav-beta3
and alphav-beta5
both of which are upregulated in tumor-associated vasculature [302].
11C- half-life is approximately 20 minutes
11C-L-methylmethionine:
11C-L-methylmethionine measures amino acid uptake/protein synthesis (tumor cells require an external supply of methionine). Methionine is needed for protein synthesis as a precursor of S-adenosylmethionine which is required for polyamine synthesis [55]. Increased uptake of methionine reflects increased carrier mediated transport, transmethylation rate, and protein synthesis in malignant tissue [55,130]. The agent has primarily been used for imaging of CNS neoplasms [18]. There is normally low uptake in the brain (gray matter) due to low amino acid metabolism in normal brain tissue, and somewhat higher uptake in the salivary glands, lacrimal glands, bone marrow, and occasionally the myocardium [18,243]. Abdominal uptake in the liver and pancreas (usually high uptake) is frequently seen, and there is variable intestinal uptake of the agent [18]. Moderate activity is seen in the renal cortex [18]. Pituitary uptake is high [18]. See also CNS tumor imaging.
11C-thymidine:
11C-thymidine is used in the evaluation of tumor DNA replication and cell division rates. 68Ga-EDTA has been used in the evaluation of disruption of the blood brain barrier.
11C-choline:
11C-choline (11C-CHOL) is an agent that is incorporated into tumor cells by conversion (a phosphorylation reaction that is catalyzed by choline kinase [261]) into 11C-phosphorlycholine which is trapped inside the cell. This is followed by synthesis of 11C-phosphatidylcholine which constitutes a main component of cell membranes [26]. Because tumor cells duplicate very quickly, the biosynthesis of cell membranes is also very fast and there is increased uptake of choline and upregulation/overexpression of the enzyme choline kinase [26,261]. Essentially, the uptake of 11C-CHOL in tumors represents the rate of tumor cell proliferation [5]. 11C-CHOL is very rapidly cleared from the blood, and optimal tumor-to-background contrast is reached within 5 minutes [26,59]. The initial high uptake remains at a nearly constant level afterward [59]. There are two mechanisms of tracer uptake: energy dependent choline-specific transport and simple diffusion [59].
Prominent tracer uptake is seen in the liver, renal cortex, and salivary glands (the liver and kidney are major sites for choline oxidation) [26]. Less intense uniform tracer uptake can be seen in then lungs, spleen, skeletal muscles, bone marrow, choroid plexus, and pituitary gland [26]. High to variable pancreatic and duodenal uptake can be seen due to secretion of phospholipid-rich pancreatic juice [26,59]. Variable uptake is seen in the small bowel and urinary bladder [26]. Generally, there is only minimal activity in the urinary tract and bladder of fasting individuals which may aid in the evaluation of pelvic malignancies [59]. No uptake is generally observed in the mediastinum or myocardium [26]. Since normal brain cells are in a non-dividing state, the uptake of 11C-CHOL is very low, while brain tumors and metastases have increased cell membrane synthesis and increased tracer uptake [59]. The agent is superior to FDG-PET imaging for the detection of brain metastases from lung cancer [26]. Tracer uptake can occur in inflammatory processes such as pelvic inflammatory disease [59].
11C-acetate is another PET tumor imaging agent. Acetate can be metabolized as a substrate for beta-oxidation through the tricarboxylic acid cycle (Krebs cycle) or it can be used as a substrate for fatty acid synthesis and the production of phospholipids in cellular membranes [179,191,261]. In the myocardial mitochondria, 11C-acetate is converted into acetyl-coenzyme A which is followed by rapid metabolism via the citric acid cycle and clearance as CO2. However, as previously mentions- acetate is not only a metabolic substrate of beta-oxidation, but also a precursor of amino acids, fatty acids, and sterol [58]. The mechanism of tumor uptake is uncertain, but may be multifactoral [57] and related to low oxygen consumption and enhanced lipid synthesis in tumor cells [88]. The agent appears to be incorporated into cell membrane lipids [160] (it may be involved with active basal lipid metabolism in the cell membrane in cancer tissue with low oxidative metabolism and high lipid synthesis [57,58]). Preliminary studies have indicated that certain prostate tumors prefer 11C-acetate to FDG as a substrate for cellular metabolism [88]. Malignancy induced upregulation of fatty acid synthase (FAS) may play a role in 11C-acetate uptake in prostate cancers [15,261]. FAS is a multifuncational enzymatic protein that catalyzes fatty acid biosynthesis- it is overexpressed in prostate cancers (as well as other tumors), but levels are low or absent in most normal tissues [15]. 11C-acetate also shows high uptake in other tumors such as renal cell carcinomas and hepatomas [160]. In the brain, acetate is preferentially metabolized by astrocytes with low uptake in normal brain tissue [225]. The agent is taken up by all meningiomas [225].
Normal biodistribution: 11C-acetate accumulates in the pancreas (high accumulation), there is variable uptake in the liver and bowel, heart, kidneys, spleen, stomach, and bone marrow [88,241]. Although there is some renal uptake, urinary excretion is not seen, although mild bladder activity may be observed [57]. The lack of urinary activity is useful for imaging prostate cancer [241]. There is relatively rapid clearance of the agent from most other tissues because of its oxidative metabolism to 11C-CO2.
Tumor hypoxia agents:
Tumor cell growth outstrips vascular development so that oxygen
delivery is decreased beyond its diffusion distance [194].
Hypoxemia promotes neovascularization through a variety of
molecular signals [194]. FDG uptake in tumors reflects tumor
hypoxia because glucose transporter expression (GLUT-1) is
up-regulated in hypoxia [282]. Tumors that demonstrate low
perfusion, but high glucose metabolism (a dissociation between
metabolism and flow) show an adaptation to hypoxia and reflects
tumor aggressiveness and may reflect a poorer response to
treatment [266]. However, tumor hypoxia and glucose metabolism do
not always correlate [282]. The intracellular partial pressure of
oxygen in normally perfused cells is in the range of 30-50 mm Hg;
while in hypoxic tumors it can be as low as 3-15 mmHg [188].
Tumor hypoxia has been shown to correlate with a worse prognosis
in certain malignancies [178,307]. Hypoxic cells are more
resistant to radiation and chemotherapy and therefore require dose
escalation radiation treatment or additional chemo- or hypoxia
directed therapy to achieve adequate cytotoxicity [74,76,134,178,262,307]. Hypoxia causes
radiotherapy resistance primarily by limiting oxygen fixation, the
crucial step for radiation to effect DNA damage [331]. The
presence of hypoxia is associated with more aggressive tumor
phenotypes, resulting in local tissue invasion and an increased
metastatic potential [192]. Hypoxia has also been shown to be
associated with increased multidrug resistance type 1 protein
(MDR1) [204]. In general, patients with tumors that have significant hypoxia have a worse
prognosis and shorter disease free survival when compared to
better oxygenated tumors [134,154]. One point to remember is that
hypoxia is a dynamic process in constant change in in the relative
contribution of acute and chronic hypoxia to the total hypoxic
tumor volume [284]. Definition of the hypoxic volume can aid in
choosing the appropriate target for radiation dose escalation
[17].
Interestingly, the agent metformin may have radiosensitizing
properties and may affect tumor response and growth either
directly or indirectly [331]. The direct effect is attributed to
the inhibition of the mitochondrial complex I and its downstream
pathways, resulting in activation of the cellular energy sensor
adenosine monophosphate-activated kinase [331]. This activation in
turn increases cellular catabolism and reduced anabolism [331].
The indirect effect is caused by metformin's lowering effects on
blood glucose and insulin levels, two major factors that stimulate
cancer growth [331].
18F-fluoromisonodazole (FMISO): Radiolabeled
nitroimidazoles can be used for the evaluation of tumor hypoxia
[134]. 18F-fluoromisonidazole (18F-FMISO)
is a PET tracer used for the evaluation of tissue hypoxia
[134,154]. 18F-FMISO acts as a bioreceptor molecule
and is incorporated into cell constituents under hypoxic
conditions [74]. The agent is lipophilic and freely diffuses
across the cell membrane and is metabolized by intracellular
nitroreductases into a radical anion (in the presence of oxygen
the agent readily diffuses back out of the cell) [194,210,304].
Oxygen acts as an electron acceptor for the 18F-FMISO
radical anion [194]. Under normoxic conditions the reduced form of
fluoromisonodazole is rapidly oxidized back to it's diffusible
form, with tissue levels rapidly declining as blood borne
fluoromisonodazole is cleared [304]. In the absence of oxygen, the
unstable 18F-FMISO radical anion is further reduced
(by nitroreductase) and covalently binds to intracellular
macromolecules and becomes trapped within the hypoxic, yet
metabolically active cells, leading to progressive accumulation of
the agent [134,188,194,246,304]. Nitroimidazoles bind to cells at
a rate that is maximal under conditions of severe hypoxia (less
than 0.05% oxygen) and are inhibited as a function of increasing
oxygen concentration [134,194]. The uptake of the agent is
increased only when tissue hypoxia of pO2 < 10
mm Hg is present [154] (and significant retention can be seen at
oxygen levels below 3 mm Hg [194]). The metabolism of the agent
requires active electron transport and therefore the agent is not
incorporated into necrotic tissue [134]. Unfortunately,
there is slow clearance from the blood compartment, slow cellular
uptake (the agent traverses tissue by passive diffusion), and slow
washout from non-hypoxic tissues which limit image quality and the
effectiveness of this agent [74,204,262]. Because
blood pool clearance of the agent is slow, a 2.5 hour wait
after injection is required to allow for adequate contrast between
hypoxic and non-hypoxic tissue [210,269]. A
longer delay of up to 4 hours has also been used [269]. Contrast
between normal and pathologic tissue can be low [246].
A FMISO tumor-to-blood activity ratio of greater than 1.2-1.4 has
been used for defining the presence of hypoxia [242,304]. This low
contrast is also in part related to the heterogeneous distribution
of hypoxic regions within tumors- often only 5-30% of a tumor is
hypoxic [262]. Note that a combination of severely hypoxic and
necrotic tissues supplied by structurally and functionally
abnormal vasculature may lead to low total uptake of the agent,
even at late time points [304]. Additionally, tissue may be
misidentified as hypoxic as a consequence of slow clearance of the
agent from regions of high tumor perfusion [320]. This variable
uptake and slow clearance means that it is not always possible to
unambiguously differentiate the impact on uptake of hypoxia and
perfusion by static PET imaging alone [320].
18F-fluoroazomycin arabinoside (18F-FAZA)
is also used to study hypoxia [316]. FAZA undergoes electron
reduction as it enters the cell [307]. Under normoxic conditions
the tracer is reoxygenated and transported out of the cell [307].
Under hypoxic conditions the tracer is retained in the cell [307].
The agent undergoes rapid renal clearance and clears from the
blood more rapidly than FMISO resulting in a more favorable
tumor-to-background ratio in most anatomic regions [282,284,307].
The agent also exhibits good in vivo stability against enzymatic
activity [282].
62Cu-ATSM (diacetyl-bis (N4-mthylthiosemicarbazone) is another tumor hypoxia agent which diffuses into cells where it is selectively bioreduced and trapped within viable cells under hypoxic conditions (the agent undergoes rapid clearance from non-hypoxic tissue) [74,76,178,209]. 62Cu has a half-life of 9.7 minutes and can be produced from a 62Zn/62Cu generator [178].
64Cu-ATSM and 64Cu-PTSM
are two other PET agents used for hypoxia imaging [204]. 64Cu-PTSM
is a PET perfusion tracer with distribution proportional to blood
flow, but the agent lacks hypoxia selectivity [204]. 64Cu-ATSM
is
highly
lipophilic
and
differs
from
PTSM
in
only
one
methyl
group,
but
it
possesses a lower redox potential- consequently it is more readily
reduced and trapped in hypoxic tissues [204]. 64Cu-ATSM
shows rapid tumor delineation and high tumor-to-blood ratios
[204]. The mechanism underlying its retention
are incompletely understood [204]. A drawback of 64Cu imaging is the emission of beta
particles (38.5%) which results in less favorable dosimetry
[316].
REFERENCES:
(1) J Nucl Med 1994; Larson SM. Cancer or inflammation? A holy grail for nuclear medicine. 35 (10):
1653-55 (No abstract available)
(2) AJR 1998; Eubank WB, et al. Imaging of oncologic patients: Benefit of combined CT and FDG PET in the diagnosis of malignancy. 171:1103-10
(3) J Nucl Med 1999; Coleman RE. PET in lung cancer. 40: 814-820
(4) J Nucl Med 1999; Delbeke D. Oncological applications of FDG PET imaging: Brain tumors, colorectal cancer, lymphoma, and melanoma. 40: 591-603
(5) J Nucl Med 2000; Hara T, et al. Sensitive detection of mediastinal lymph node metastasis of lung cancer with 11C-Choline PET. 41: 1507-1513
(6) Radiology 1997; Moog F, et al. Lymphoma: role of whole-body 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) PET in nodal staging. 203: 795-800
(7) Blood 1999; Jerusalem BG, et al. Whole-body positron emission tomography using 18-F-Fluorodeoxyglucose for posttreatment evaluation in Hodgkin's disease and non-Hodgkin's lymphoma has higher diagnostic and prognostic value than classical conventional tomography scan imaging. 94 (2): 429-433 (No abstract available)
(8) Acta Oncol 1999; Bangerter M, et al. Positron emission tomography with 18-Fluorodeoxyglucose in the staging and follow-up of lymphoma in the chest. 38 (6): 799-804
(9) J Nucl Med 1999; Moog F, et al. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. 40 (9): 1407-13
(10) The normal PET scan. von Schulthess GK, et al. In- Clinical positron emission tomography. Correlation with morphological cross-sectional imaging. Ed. von Schulthess GK. Lippincott Williams & Wilkins. 2001: 49-69.
(11) Radiographics 2000; Skehan S, et al. Imaging features of primary and recurrent esophageal cancer at FDG PET. 20: 713-723
(12) J Nucl Med 2000; Higashi T, et al. Evaluation of the early effect of local irradiation on normal rodent bone marrow metabolism using FDG: Preclinical PET studies. 41: 2026-2035
(13) J Nucl Med 2000; Ballinger JR. Local and distant effects of radiotherapy on FDG accumulation in bone marrow. 41: 2036-37 (No abstract available)
(14) Thorax 1998; Lowe VJ, Naunheim KS. Current role of positron emission tomography in thoracic oncology. 53: 703-712
(15) Ann Thorac Surg 1998; Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. 65: 1821-29
(16) J Nucl Med 2001; Avril N, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. 42: 9-16
(17) J Nucl Med 2001; Dimitrakopoulou-Strauss A, et al. Quantitative PET studies in pretreated melanoma patients: A comparison of 6-[18F] Fluoro-L-Dopa with 18F-FDG and 15O-water using compartment and noncompartment analysis. 42: 248-256
(18) J Nucl Med 2001; Jager PL, et al. Radiolabeled amino acids: Basic aspects and clinical applications in oncology. 42: 432-445
(19) J Nucl Med 2001; Brink I, et al. Increased metabolic activity in the thymus gland studied with 18F-FDG PET: Age dependency and frequency after chemotherapy. 42: 591-595
(20) J Cancer Res Clin Oncol 2000; Ilknur AK, et al. Positron emission tomography with 2-[18F] fluoro-2-deoxy-D-glucose in oncology: Part II: The clinical value in detecting and staging primary tumours. 126: 560-574
(21) J Nucl Med 2001; Hicks RJ, et al. Pattern of uptake and excretion of 18F-FDG in the lactating breast. 42: 1238-1242
(22) J Nucl Med 2001; Higashi K, et al. Comparison of [18F]FDG PET and 201Tl SPECT in evaluation of pulmonary nodules. 42: 1489-1496
(23) J Nucl Med 2001; Mochizuki T, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. 42: 1551-1555
(24) Radiol Clin N Am 2001; Hagge RJ, et al. Positron emission tomography. Brain tumors and lung cancer. 39: 871-881
(25) J Nucl Cardiol 2001; Dilsizian V, et al. Fluorine-18-deoxyglucose SPECT and coincidence imaging for myocardial viability: Clinical and technical issues. 8: 75-88 (No abstract available)
(26) J Nucl Med 2002; Pieterman R, et al. Comparison of 11C-choline and 18F-FDG PET in primary diagnosis and staging of patients with thoracic cancer. 43: 167-172
(27) J Nucl Med 2002; Higashi T, et al. Relationship between retention index in dual-phase 18F-FDG PET , and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. 43: 173-180
(28) J Clin Oncol 2002; Bos R, et al. Biologic correlates of 18Fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. 20: 379-387
(29) Radiology 2002; Kamel EM, et al. Recurrent laryngeal nerve palsy in patients with lung cancer: detection with PET-CT image fusion- report of six cases. 224: 153-156
(30) AJR 2002; Dizendorf EV, et al. Application of oral contrast media in coregistered positron emission tomography- CT. 179: 477-481
(31) Surgery 2001; Cohen MS, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. 130: 941-946
(32) J Nucl Med 1999; Sugawara Y, et al. Splenic fluorodeoxyglucose uptake increased by granulocyte colony-stimulating factor therapy: PET imaging results. 40: 1456-62
(33) Clin Nucl Med 1999; Abdel-Dayem HM, et al. Fluorine-18 deoxyglucose splenic uptake from extramedullary hematopoiesis after granulocyte colony-stimulating factor stimulation. 24: 319-322
(34) J Nucl Med 2002; Jager PL. Improving amino acid imaging: hungry or stuffed? 43: 1207-1209 (No abstract available)
(35) J Nucl Med 2002; Rasey JS, et al. Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. 43: 1210-1217
(36) J Nucl Med 2002; Matthies A, et al. Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. 43: 871-875
(37) Radiographics 1998; Frasmus JJ, et al. Thoracic FDG PET: state of the art. 18: 5-20.
(38) J Clin Oncol 1998; Lowe VJ, et al. Prospective investigation of positron emission tomography in lung nodules. 16: 1075-84
(39) GE PET Masters Series Clinical PET; Delbeke D. Verbal communication. Sept 18-20, 2002
(40) Radiology 2002; Tatlidil R, et al. Incidental colonic fluorodeoxyglucose uptake: correlation with colonoscopic and histopathologic findings. 224: 783-787
(41) AJR 2002; Goerres GW, et al. Positron emission tomography and PET CT of the head and neck: FDG uptake in normal anatomy, in benign lesions, and in changes resulting from treatment. 179: 1337-1343 (No abstract available)
(42) Radiology 1999; Marom EM, et al. Staging non-small cell lung cancer with whole-body PET. 2121: 80-809
(43) Ann Thorac Surg 1999; Saunders CAB, et al. Evaluation of fluorodeoxyglucose whole body positron emission tomography in the staging of lung cancer. 67: 790-97
(44) Radiol Clin N Am 2001; Hagge RJ, et al. Positron emission tomography. Brain tumors and lung cancer. 39: 871-881
(45) Radiology 2003; Rohren EM, et al. Screening for cerebral metastases with FDG PET in patients undergoing whole-body staging of non-central nrevous system malignancy. 226: 181-187
(46) Radiology 1993; Griffeth LK, et al. Brain metastases from non-central nervous system tumors: evaluation with PET. 186: 37-44
(47) J Nucl Med 2003; Dizendorf EV, et al. Impact of whole-body 18F-FDG PET on staging and managing patients for radiation therapy. 44: 24-29
(48) AJR 2003; Wittram C, et al. Thymic enlargement and FDG uptake in three patients: CT and FDG positron emission tomography correlated with pathology. 180: 519-522
(49) Eur J Nucl Med Mol Imaging 2002; Zhuang H, et al. Persistent non-specific FDG uptake on PET imaging following hip arthroplasty. 29:1328-33
(50) Nucl Med Commun 2002; Chacko TK, et al. The importance of the location of fluorodeoxyglucose uptake in periprosthetic infection in painful hip prostheses. 23:851-5
(51) Radiology 2003; Goerres GW, et al. Artifacts at PET and PET/CT caused by metallic hip prosthetic material. 226: 577-584
(52) J Nucl Med 2003; Cohade C, et al. Uptake in supraclavicular are fat ("USA-fat") description on 18F-FDG PET/CT. 44: 170-176
(53) J Nucl Med 2003; Kostakoglu L, Goldsmith SJ. 18F-FDG PET evaluation of the response to therapy for lymphoma and for breast, lung, and colorectal carcinoma. 44: 224-239
(54) Radiographics 2003; Kostakoglu L, et al. Clinical role of FDG PET in evaluation of cancer patients. 23: 315-340
(55) J Nucl Med 2003; Chesnay E, et al. Early response to chemotherapy in hypopharyngeal cancer: assessment with 11C-methionine PET, correlation with morphologic response, and clinical outcome. 44: 526-532
(56) J Nucl Med 2003; Hustinx R, et al. Whole-body tumor imaging using PET and 2-18F-fluoro-L-tyrosine: preliminary evaluation and comparison with FDG. 44: 533-539
(57) J Nucl Med 2003; Oyama N, et al. 11C-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. 44: 549-555
(58) J Nucl Med 2003; Dimitrakopoulou-Strauss A, Strauss L. PET imaging of prostate cancer with 11C-acetate. 44: 556-558 (No abstract available)
(59) J Nucl Med 2003; Torizuka T, et al. Imaging gynecologic tumors: comparison of 11C-choline PET with 18F-FDG PET. 44: 1051-1056
(60) AJR 2003; Kalra MK, et al. Correlation of positron emission tomography and CT in evaluating pancreatic tumors: technical and clinical implications. 181: 387-393 (No abstract available)
(61) J Nucl Med 2003; Vranjesevic D, et al. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. 44: 1238-1242
(62) J Nucl Med 2003; Cohade C, et al. "USA-fat": prevalence is related to ambient outdoor temperature- evaluation with 18F-FDG PET/CT. 44: 1267-1270
(63) J Nucl Med 2003; Buck AK, et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. 44: 1426-1431
(64) J Nucl Med 2003; Shields AF. PET imaging with 18F-FLT and thymidine analogs: promise and pitfalls. 44: 1432-1434 (No abstract available)
(65) J Nucl Med 2003; Vesselle H, et al. 18F-fluorothymidine radiation dosimetry in human PET imaging studies. 44: 1482-1488
(66) AJR 2003; Fayad LM, et al. Sacral fractures: a potential pitfall of FDG positron emission tomography. 181: 1239-1243
(67) J Nucl Med 2003; Yeung HW, et al. Patterns of 18F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. 44: 1789-1796
(68) Radiology 2003; Tatsumi M, et al. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. 229: 831-837
(69) J Nucl Med 2003; Cobben DCP, et al. 3'-18F-fluoro-3'-deoxy-L-thymidine: a new tracer for staging metastatic melanoma? 44: 1927-1932
(70) J Nucl Med 2003; Schwartz JL, et al. Monitoring tumor cell proliferation by targeting DNA synthetic processes with thymidine and thymidine analogs. 44: 2027-2032
(71) AJR 2004; Nguyen M, et al. CT with histopathologic correlation of FDG uptake in a patient with pulmonary granuloma and pleural plaque caused by remote talc pleurodesis. 182: 92-94 (No abstract available)
(72) AJR 1997; Murray JG, et al. Talc pleurodesis simulating pleural metastases on 18F-fluorodeoxyglucose positron emission tomography. 168: 359-360 (No abstract available)
(73) AJR 2004; Yoshida Y, et al. Incremental benefit of FDG positron emission tomography over CT alone for the preoperative staging of ovarian cancer. 182: 227- 233
(74) J Nucl Med 2004; Kostakoglu L, Goldsmith SJ. PET in the assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. 45: 56-68
(75) Radiology 2004; Agress H, Cooper BZ. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: histopathologic comparison. 230: 417-422
(76) J Nucl Med 2004; Bradley JD, et al. Implementing biologic target volumes in radiation treatment planning for non-small cell lung cancer. 45 (Suppl): 96S-101S
(77) J Nucl Med 2004; Lerman H, et al. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. 45: 266-271
(78) J Nucl Med 2004; Even-Sapir E, et al. Assessment of malignant skeletal disease: initial experience with 18F-Fluoride PET/CT and comparison between 18F-Fluoride PET and 18F-Fluoride PET/CT. 45: 272-278
(79) Ann Pharmacother 2002; Mayer D, Bednarczyk EM. Interaction of colony stimulating factors and fluorodeoxyglucose F18 positron emission tomography. 36: 1796-99
(80) AJR 2004; Aberg C, et al. FDG positron emission tomography of bone involvement in sarcoidosis. 182: 975-977 (No abstract available)
(81) AJR 2004; Asad S, et al. False-positive FDG positron emission tomography uptake in nonmalignant chest abnormalities. 182: 983-989 (No abstract available)
(82)
(83) J Nucl Med 2004; Schoder H, et al. Clinical implications of different image reconstruction parameters for interpretation of whole-body PET studies in cancer patients. 45: 559-566
(84) J Nucl Med 2004; van Waarde A, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. 45. 695-700
(85) Radiology 2004; Rohren EM, et al. Clinical applications of PET in oncology. 231: 305-332
(86) J Nucl Med 2004; Tatsumi M, et al. Intense 18F-FDG uptake in brown fat can be reduced pharmacologically. 45: 1189-1193
(87) J Nucl Med 2004; Becherer A, et al. Imaging of advanced neuroendocrine tumors with 18F-fluoro-DOPA PET. 45: 1161-1167
(88) J Nucl Med 2004; Seltzer MA, et al. Radiation dose estimates in humans for 11C-acetate whole-body PET. 45: 1233-1236
(89) J Nucl Med 2004; Ogawa M, et al. 18F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. 45: 1245-1250
(90) J Nucl Med 2004; Bagheri B, et al. Characterization of the normal adrenal gland with 18F-FDG PET/CT: 45: 1340-1343
(91) AJR 2004; Truong MT, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. 183: 1127-1132
(92) AJR 2004; Kavanagh PV, et al. Nonneoplastic diseases in the chest showing increased activity on FDG PET. 183: 1133-1141 (No abstract available)
(93) AJR 2004; Pandit-Taskar N, et al. Clinical significance of unexplained abnormal focal FDG uptake in the abdomen during whole-body PET. 183: 1143-1147
(94) J Nucl Med 2004; Cobben DC, et al. Is 18F-3'-fluoro-3'-deoxy-L-thymidine useful for staging and restaging non-small cell lung cancer? 45: 1677-1682
(95) Nucl Med 2004; Kumar R, et al. 18F-FDG PET for evaluation of the treatment response in patients with gastrointestinal tract lymphomas. 45: 1796-1803
(96) J Nucl Med 2004; Kamel EM, et al. Significance of incidental 18F-FDG accumulations in the gastrointestinal tract in PET/CT: correlation with endoscopic and histopathologic results. 45: 1804-1810
(97) J Nucl Med 2004; Ben-Haim S, et al. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. 45: 1816-1821
(98) Radiographics 2004; Ferdinand B, et al. Spectrum of thymic uptake at 18F-FDG PET. 24: 1611-1616
(99) J Nucl Med 2005; Salaun PY, et al. An analysis of the 18F-FDG uptake pattern in the stomach. 46: 48-51
(100) J Nucl Med 2005; Waldherr C, et al. Monitoring antiproliferative responses to kinase inhibitor therapy in mice with 3'-deoxy-3'-18F-fluorothymidine PET. 46: 114-120
(101) AJR 2005; Fan CM, et al. Lipomatous hypertrophy of the interatrial septum: increased uptake on FDG PET. 184: 339-342
(102) J Nucl Med 2005; Muzi M, et al. Kinetic analysis of 3'-deoxy-3'-fluorothymidine PET studies: validation studies in patients with lung cancer. 46: 274-282
(103) Radiology 2005; Nakamoto Y, et al. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. 234: 879-885
(104) Radiographics 2005; Kazama T, et al. FDG PET in the evaluation of treatment for lymphoma: clinical usefulness and pitfalls. 25: 191-207
(105) J Nucl Med 2005; van Westreenen HL, et al. Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. 46: 400-406
(106) J Nucl Med 2005; PET with O-(2-18F-Fluoroethyl)-L-tyrosine in peripheral tumors: first clinical results. 46: 411-416
(107) J Nucl Med 2005; Jaskowiak CJ, et al. Influence of reconstruction iterations on 18F-FDG PET/CT standardized uptake values. 46: 424-428
(108) AJR 2005; Aide N, et al. Persistent foreign body reaction around inguinal mesh prostheses: a potential pitfall of FDG PET. 184: 1172-1177
(109) Radiol Clin N Am 2004; Alavi A, et al. PET: a revolution in medical imaging. 42: 983-1001
(110) Radiol Clin N Am 2004; Acton PD, et al. Quantification in PET. 42: 1055-1062
(111) Radiol Clin N Am 2004; El-Haddad G, et al. Normal variants in [18F]-fluorodeoxyglucose PET imaging. 42: 1063-1081
(112) Radiol Clin N Am 2004; Zhuang H, et al. Investigation of thyroid, head, and neck cancers with PET. 42: 1101-1111
(113) J Nucl Med 2005; Ishimori T, et al. Detection of unexpected additional primary malignancies with PET/CT. 46: 752-757
(114) J Nucl Med 2005; Israel O, et al. PET/CT detection of unexpected gastrointestinal foci of 18F-FDG uptake: incidence, loclaization, patterns, and clinical signifincace. 46: 758-762
(115) J Nucl Med 2005; Kobayashi Y, et al. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. 46: 917-922
(116) J Nucl Med 2005; Yun M, et al. The role of gastric distention in differentiating recurrent tumor from physiologic uptake in the remnant stomach on 18F-FDG PET: 46: 953-957
(117) J Nucl Med 2005; Chen W, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. 46: 945-952
(118) J Nucl Med 2005; Weber WA. Use of PET for monitoring cancer therapy and predicting outcome. 46: 983-995
(119) J Nucl Cardiol 2005; Tawakol A, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. 12: 294-301
(120) Radiology 2005; Geun J, et al. Focal uptake of fluorodeoxyglucose by the thyroid in patients undergoing initial disease staging with combined PET/CT for non-small cell lung cancer. 236: 271-275
(121) J Nucl Med 2005; Dunphy MPS, et al. Association of vascular 18F-FDG uptake with vascular calcification. 46: 1278-1284
(122) J Nucl Med 2005; Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. 46: 1356-1367
(123) AJR 2005; Gutman F, et al. Incidental colonic focal lesions detected by FDG PET/CT. 185: 495-500
(124) AJR 2005; Wandler E, et al. Diffuse FDG shoulder uptake on PET is associated with clinical findings of osteoarthritis. 185: 797-803
(125) Radiol Clin N Am 2005; Jadvar H, et al. PET in pediatric diseases. 43: 135-152
(126) Radiol Clin N Am 2005; Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. 43: 189-204
(127) Radiographics 2005; Blodgett TM, et al. Combined PET-CT in the head and neck. Part 1. Physiologic, altered physiologic, and artifactual FDG uptake. 25: 897-912
(128) J Nucl Med 2005; Kumar R, et al. Potential of dual-time-point imaging to improve breast cancer diagnosis with 18F-FDG PET. 46: 1819-1824
(129) J Nucl Med 2005; Pandit-Taskar N. Oncologic imaging in gynecologic malignancies. 46: 1842-1850
(130) J Nucl Med 2005; Jacobs AH, et al. 18F-fluoro-L-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. 46: 1948-1958
(131) AJR 2006; Mawlawi O, et al. Quantifying the effect of IV contrast media on integrated PET/CT: clinical evaluation. 186: 308-319
(132) J Nucl Med 2006; Pauleit D, et al. 18F-FET-PET compared with 18F-FDG-PET and CT in patients with head and neck cancer. 47: 256-261
(133) J Nucl Med 2006; Even-Sapir E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. 47: 287-297
(134) J Nucl Med 2006; Cher LM, et al. Correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in gliomas using 18F-fluoromisonidazole, 18F-FDG PET, and immunohistochemical studies. 47: 410-418
(135) J Nucl Med 2001; Yun M, et al. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. 42: 1795-1799
(136) J Nucl Med 2006; Metser U, et al. 18F-FDG PET/CT in the evaluation of adrenal masses. 47: 32-37
(137) Radiology 2006; Blake MA, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy- initial experience. 238: 970-977
(138) Chest 2006; Yap CS, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. 129: 393-401
(139) J Nucl Med 2006; Meisetschlager G, et al. Gluc-Lys ([18F]FP)-TOCA PET in patients with SSTR-positive tumors: biodistribution and diagnostic evaluation compared with [111In]DTPA-octreotide. 47: 566-573
(140) J Nucl Med 2006; Choi JY, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. 47: 609-615
(141) J Nucl Med 2006; Chen W, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. 47: 904-911
(142) J Nucl Med 2006; Jacene HA, et al. Effects of pegfilgrastim on normal biodistribution of 18F-FDG: preclinical and clinical studies. 47: 950-956
(143) J Nucl Med 2006; Shankar LK, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in national cancer institute trials. 47: 1059-1066
(144) AJR 2006; Korn RL, et al. Unexpected focal hypermetabolic activity in the breast: significance in patients undergoing 18F-FDG PET/CT. 187: 81-85
(145) J Nucl Med 2006; Avril N. 18F-FDG PET after radiofrequency ablation: is timing everything? 47: 1235-1237
(146) J Nucl Med 2006; Rosen RS, et al. Increased 18F-FDG uptake in degenerative disease of the spine: characterization with 18F-FDG-PET/CT. 47: 1274-1280
(147) J Nucl Med 2006; Jhanwar Y, Straus DJ. The role of PET in lymphoma. 47: 1326-1334
(148) J Nucl Med 2006; Montravers F, et al. Can fluorodihydroxyphenylalanine PET replace somoatostatin receptor scintigraphy in patients with digestive endocrine tumors? 47: 1455-1462
(149) AJR 2006; Iagaru A, Henderson R. PET/CT followup in nonossifying fibroma. 187: 830-832
(150) AJR 2006; Goodin GS, et al. PET/CT characterization of fibroosseous defects in children: 18F-FDG uptake can mimic metastatic disease. 187: 1124-1128
(151) J Nucl Med 2006; Agool A, et al. 18F-FLT PET in hematologic disorders: a novel technique to analyze the bone marrow compartment. 47: 1592-1598
(152) J Nucl Med 2006; Muzi M, et al. Kinetic analysis of 3'-deoxy-3'-18F-fluorothymidine in patients with gliomas. 47: 1612-1621
(153) J Nucl Med 2006; Kamel EM, et al. Forced diuresis improved the diagnostic accuracy of 18F-FDG-PET in abdominopelvic malignancies. 47: 1803-1807
(154) J Nucl Med 2006; Cherk MH, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG-PET. 47: 1921-1926
(155) Radiographics 2006; Chong S, et al. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnosic efficacy and interpretation pitfalls. 26: 1811-1826
(156) Radiographics 2007; Prabhakar HB, et al. Bowel hot spots at PET-CT. 27: 145-159
(157) J Nucl Med 2007; Roh JL, et al. Clinical utility of 18F-FDG-PET for patients with salivary gland malignancies. 48: 240-246
(158) J Nucl Med 2007; Kasamon YL, et al. Integrating PET and PET/CT into risk-adapted therapy of lymphoma. 48: 19S-27S
(159) J Nucl Med 2007; Weber WA, Figlin R. Monitoring cancer treatment with PET/CT: does it make a difference? 48: 36S-44S
(160) J Nucl Med 2007; Ponde DE, et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging- in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. 48: 420-428
(161) J Nucl Med 2007; Gabriel M, et al. 68Ga-DOTA-Tyr3-Octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. 48: 508-518
(162) J Nucl Med 2007; Schillaci O. Somatostatin receptor imaging in patients with neuroendocrine tumors: not only SPECT? 48: 498-500
(163) J Nucl Med 2007; Floeth FW, et al. Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. 48: 519-527
(164) J Nucl Med 2007; Rutten I, et al. PET/CT of skull base meningiomas using 2-18F-fluoro-L-tyrosine: initial report, 48: 720-725
(165) Radiographics 2007; Elaini AB, et al. Improved detection and characterization of adrenal disease with PET-CT. 27: 755-767
(166) J Nucl Med 2007; Karatanis D, et al. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. 48: 896-901
(167) J Nucl Med 2007; Doot RK, et al. Dynamic and static approaches to quantifying 18F-FDG uptake for measuring cancer response to therapy, including the effect of granulocyte CSF. 48: 920-925
(168) J Nucl Med 2007; Baba S, et al. Effect of nicotine and ephedrine on the accumulation of 18F-FDG in brown adipose tissue. 48: 981-986
(169) AJR 2007; Smith CS, et al. Thymic extension in the superior mediastinum in patients with thymic hyperplasia: potential cause of false-positive findings on 18F-FDG PET/CT. 188: 1716-1721
(170) J Nucl Med 2007; Louis E, et al. Noninvasive assessment of Crohn's disease intestinal lesions with 18F-FDG PET/CT. 48: 1053-1059
(171) AJR 2007; Wittram C, Scott JA. 18F-FDG PET of pulmonary embolism. 189: 171-176
(172) J Nucl Med 2007; Nanni C, et al. 18F-DOPA PET and PET/CT. 48: 1577-1579
(173) J Nucl Med 2007; Timmers HJ, et al. The effects of crbiopa on uptakeof 6-18F-fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma.48: 1599-1606
(174) J Nucl Med 2007; Baba S, et al. Comparison of uptake of multiple clinical radiotracers into brown adipose tissue under cold-stimulated and nonstimulated conditions. 48: 1715-1723
(175) J Nucl Med 2007; Zhernosekov KP, et al. Processing of generator-produced 68Ga for medical application. 48: 1741-1748
(176) Radiographics 2006; Blake MA, et al. Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT: 26: 1335-1353
(177) J Nucl Med 2008; Grant FD, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. 49: 68-78
(178) AJR 2008; Wong TZ, et al. PET of hypoxia and perfusion with 62Cu-ATSM and 62Cu-PTSM using a 62Zn/62Cu generator. 190: 427-432
(179) J Nucl Med 2008; Vavere AL, et al. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. 49: 327-334
(180) J Nucl Med 2008; Hsu WK, et al. Characterization of osteolytic, osteoblastic, and mixed lesions in a prostate cancer mouse model using 18F-FDG and 18F-fluoride PET/CT. 49: 414-421
(181) AJR 2008; Smith CS, et al. False-positive findings on 18F-FDG PET/CT: differentiation of hibernoma and malignant fatty tumor on the basis of fluctuating standardized uptake values. 190: 1091-1096
(182) J Nucl Med 2008; Jager PL, et al. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. 49: 573-586
(183) AJR 2008; Williams G, Kolodny GM. Method for decreasing uptake of 18F-FDG by hypermetabolic brown adipose tissue on PET. 190: 1406-1409
(184) AJR 2008; Roedl JB, et al. Visual PET/CT scoring for nonspecific 18F-FDG uptake in the differentiation of early malignant and benign esophageal lesions. 191: 515-521
(185) AJR 2008; Kwak JY, et al. Thyroid incidentalomas identified by 18F-FDG PET: sonographic correlation. 191: 598-603
(186) J Nucl Med 2008; Lee SJ, et al. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. 49: 1277-1282
(187) J Nucl Med 2008; Karam M, et al. Bilateral hilar foci of 18F-FDG PET scan in patients without lung cancer: variables associated with benign and malignant etiology. 49: 1429-1436
(188) J Nucl Cardiol 2008; Jain D, He ZX. Direct imaging of myocardial ischemia: a potential new paradigm in nuclear cardiovascular imaging. 15: 617-630
(189) J Nucl Med 2008; High 18F-FDG uptake in synthetic aortic vascular grafts on PET/CT in symptomatic and asymptomatic patients. 49: 1601-1605
(190) AJR 2008; Vikram R, et al. Utility of PET/CT in differentiating benign from malignant adrenal nodules in patients with cancer. 191: 1545-1551
(191) J Nucl Med 2008; Park JW, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. 49: 1912-1921
(192) J Nucl Med 2008; Komar G, et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. 49: 1944-1951
(193) AJR 2009; Iyer RB, et al. Adrenal pheochromocytoma with surronding brown fat stimulation. 192: 300-301
(194) J Nucl Med 2009; Swanson KR, et al. Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. 50: 36-44
(195) J Nucl med 2009; Roy FN, et al. Impact of intravenous insulin on 18F-FDG PET in diabetic cancer patients. 50: 178-183
(196) J Nucl Med 2009; Zhao B, et al. Imaging surrogates of tumor response to therapy: anatomic and functional biomarkers. 50: 239-249
(197) AJR 2009; Nakajo M, et al. Effect of clinicopathologic factors on visibility of colorectal polyps with FDG PET. 192: 754-760
(198) AJR 2009; Boland GW, et al. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative versus quantitative accuracy in 150 consecutive patients. 192: 956-962
(199) J Nucl Med 2009; Fox JJ, Strauss HW. Onestep closer to imaging vulnerable plaque in the coronary arteries. 50: 497-500
(200) J Nucl Med 2009; Iagaru A, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: results of the pilot phase study. 50: 501-505
(201) J Nucl Med 2009; Groves AM, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. 50: 538-545
(202) J Nucl Med 2009; Wykrzykowska J, et al. Imaging of inflammed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. 50: 563-568
(203) J Nucl Med 2009; Jerushalmi J, et al. Physiologic thymic uptake of 18F-FDG in children and young adults: a PET/CT evaluation of incidence, patterns, and relationship to treatment. 50: 849-853
(204) J Nucl Med 2009; Liu J, et al. Retention of the radiotracers 64Cu-ATSM and 64Cu-PTSM in human and murine tumors is influenced by MDR1 protein expression. 50: 1332-1339
(205) AJR 2009; Paidisetty S, Blodgett TM. Brown fat: atypical locations and appearances encountered in PET/CT. 193: 359-366
(206) J Nucl Med 2009; Jacene HA, et al. Assessment of interobserver reproducibility in quantitative 18F-FDG PET and CT measurements of tumor response to therapy. 50: 1760-1769
(207) J Nucl Med 2009; Jadvar H, et al. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. 50: 1820-1827
(208) J Nucl Med 2009; Kayani I, et al. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. 50: 1927-1932
(209) J Nucl Med 2009; Lohith TG, et al. Pathophysiologic correlation between 62Cu-ATSM and 18F-FDG in lung cancer. 50: 1948-1953
(210) J Nucl Med 2010; Wang W, et al. Pharmacokinetic analysis of hypoxia 18F-fluoromisonidazole dynamic PET in head and neck cancer. 51: 37-45
(211) J Nucl Med 2010; Visser EP, et al. SUV: silly useless value to smart uptake value. 51: 173-175
(212) J Nucl Med 2010; Rominger A, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. 51: 193-197
(213) J Nucl Med 2010; Baba S, et al. CT hounsfield units of brown adipose tissue increase with activation: preclinical and clinical studies. 51: 246-250
(214) AJR 2010; Iyer VR, Lee SI. MRI, CT, and PET/CT for ovarian cancer detection and adenexal lesion characterization. 194: 311-321
(215) J Nucl Med 2010; Mavi A, et al. The effect of age, menopausal state, and breast density on 18F-FDG uptake in normal glandular breast tissue. 51: 347-352
(216) J Nucl Med 2010; Campana D, et al. Standardized uptake values of 68Ga-DOTANOC PET: a promising prognostic tool in enuroendocrine tumors. 51: 353-359
(217) J Nucl Med 2008; Qazi F, et al. Fatty liver: impact on metabolic activity as detected with 18F FDG-PET/CT. 49 (meeting abstract- supplement 1) 236P
(218) Radiology 2010; Abele JT, Fung CI. Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values. 254: 917-924
(219) Radiology 2010; Drubach LA, et al. Skeletal trauma in child abuse: detection with 18F-NaF PET. 255: 173-181
(220) Radiology 2010; Davison JM, et al. Squamous cell carcinoma of the palatine tonsils: FDG standardized uptake value ratio as a biomarker to differentiate tonsillar carcinoma from physiologic uptake. 255: 578-585
(221) J Nucl Cardiol 2010; Cheng VY, et al. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: a randomized controlled trial. 17: 286-291
(222) AJR 2010; Kei PL, et al. Incidental finding of focal FDG uptake in the bowel during PET/CT: CT features and correlation with hsitopathologic results. 194: 1304
(223) J Nucl Med 2010; Yun M, et al. Physiologic 18F-FDG uptake in the fallopian tubes at mid cycle on PET/CT. 51: 682-685
(224) J Nucl Med 2010; Weber WA. Monitoring tumor response to therapy with 18F-FLT PET. 51: 841-844
(225) AJR 2010; Han YM, et al. Diagnostic value of CT density in patients with diffusely increased FDG uptake in the thyroid gland on PET/CT images. 195: 223-228
(226) AJR 2010; Adams MC, et al. A systemic review of the factors affecting accuracy of SUV measurements. 195: 310-320
(227) AJR 2010; Kitajima K, et al. Spectrum of FDG PET/CT findings of uterine tumors. 195: 737-743
(228) Radiographics 2010; Son H, et al. PET/CT evaluation of cervical cancer: spectrum of disease. 30: 1251-1268
(229) J Nucl Med 2010; De Saint-Hubert M, Mottaghy FM. Can evaluation of targeted therapy in oncology be improved by means of 18F-FLT? 51: 1499-1500
(230) J Nucl Med 2010; Lodge MA, et al. Characterization of a perirectal artifact in 18F-FDG PET/CT. 51: 1501-1506
(231) J Nucl Med 2010; Fueger BJ, et al. Correlation of 6-18F-fluoro-L-Dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas. 51: 1532-1538
(232) J Nucl Med 2010; Segall G, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. 51: 1813-1820
(233) Eur J Nucl Med Mol Imaging 2008; Gontier E, et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. 35: 95-99
(234) Eur J Nucl Med Mol Imaging 2010; Ozulker T, et al. Clearance of the high intestinal (18)F-FDG uptake associated with metformin after stopping the drug. 37: 1011-1017
(235) AJR 2010; Osman MM, et al. 18F-FDG PET/CT of patients with cancer: comparison of whole-body and limited whole-body technique. 195: 1397-1403
(236) AJR 2010; Oh JR, et al. Impact of medication discontinuation on increased intestinal FDG accumulation in diabetic patients treated with metformin. 195: 1404-1410
(237) J Nucl Med 2010; Czernin J, et al. Molecular mechanisms of bone 18F-NaF deposition. 51: 1826-1829
(238) J Nucl Med 2010; Hofman MS, Hicks RJ. Restaging: should we PRECIST without pattern recognition? 1830-1831
(239) J Nucl Med 2010; Ambrosini V, et al. 68Ga-DOTANOC PET/CT allows somatostatin receptor imaging in idiopathic pulmonary fibrosis: preliminary results. 51: 1950-1955
(240) AJR 2011; Abikhzer G, et al. Altered hepatic metabolic activity in patients with hepatic steatosis on FDG PET/CT. 196: 176-180
(241) J Nucl Med 2011; Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. 52: 81-89
(242) J Nucl Med 2011; Chitneni SK, et al. Molecular imaging of hypoxia. 52: 165-168
(243) J Nucl Med 2011; Nagata T, et al. Examination of 11C-methionine metabolism by the standardized uptake value in the normal brain of children. 52: 201-205
(244) J Nucl Med 2011; Hyun SH, et al. Incidental focal 18F-FDG
uptake in the pituitary gland: clinical significance and
differential diagnosis. 52: 547-550
(245) J Nucl Cardiol 2011; Minamimoto R, et al. Value of
FDG-PET/CT using unfractionated heparin for managing primary
cardiac lymphoma and several key findings. 18: 516-520
(246) J Nucl Med 2011; Hugonnet F, et al. Metastatic renal cell
carcinoma: relationship between intial metastasis hypoxia, change
after 1 month's Sunitinib, and therapeutic response: an 18F-fluoromisonidazole
PET/CT study. 52: 1048-1055
(247) AJR 2011; Kang BJ, et al. Clinical significance of
incidental finding of focal activity in the breast at 18F-FDG PET/CT. 197: 341-347
(248) J Nucl Cardiol 2011; Small GR, Ruddy TD. PET imaging of
aortic atherosclerosis: is combined imaging of plaque and function
an amaranthine quest or conceivable reality? 18: 717-728
(249) AJR 2011; Drubach LA, et al. Skeletal scintigraphy with 18F-NaF PET for the evaluation of bone
pain in children. 197: 713-719
(250) Radiographics
2011;
Stolzmann P, et al. Complementary value of cardiac FDG PET and
CT for the characterization of atherosclerotic disease. 31:
1255-1269
(251) Radiographics 2011; Maurer AH, et al.
How to differentiate benign versus malignant cardiac and
paracardiac 18F FDG
uptake at oncologic PET/CT. 31: 1287-1305
(252) J Nucl Med 2011; Benz MR, et al.
18F- FDG PET/CT for monitoring
treatment responses to the epidermal growth factor inhibitor
erlotinib. 52: 1684-1689
(253) J Nucl Med 2011; Hatt M, et al. Impact
of tumor size and tracer uptake heterogeneity in 18F- FDG PET and CT non-small celll
lung cancer tumor delineation. 52: 1690-1697
(254) J Nucl Med 2012; Boellaard R. Mutatis
mutandis: harmonize the standard. 53: 1-3
(255) J Nucl med 2012; Vanderhoek M, et al.
IMpact of the definition of peak standardized uptake value on
quantification of treatment response. 53: 4-11
(256) J Nucl Med 2012; Chang KP, et
al. Prognostic significance of 18F-FDG PET parameters
and plasma Epstine-Barr virus DNA load in patients with
nasopahryngeal carcinoma. 53: 21-28
(257) Radiology 2012; Gawande RS, et al. Differentiation of
normal thymus from anterior mediastinal lymphoma and lymphoma
recurrence at pediatric PET/CT. 262: 61
(258) J Nucl Med 2012; Dibble EH, et al. 18F-FDG
metabolic tumor volume and total glycolytic activity of oral
cavity and oropharyngeal squamous cell cancer: adding value of
clinical staging. 53: 709-715
(259) Radiology 2012; Cronin CG, et al. Brown fat at PET/CT:
correlation with patient characteristics. 263: 836-842
(260) J Nucl Med 2012; Lodge MA, et al. Noise considerations for
PET quantification using maximum and peak standardized uptake
value. 53: 1041-1047
(261) AJR 2012; Jadvar H. Molecular imaging of prostate cancer:
PET radiotracers. 199: 278-291
(262) J Nucl Med 2012; Carlin S, Humm JL. PET of hypoxia: current
and future perspectives. 53: 1171-1174
(263) J Nucl Med 2012; Kurdziel KA, et al. The kinetics and
reproducibility of 18F-sodium fluoride for oncology
using current PET camera technology. 53: 1175-1184
(264) J Nucl Med 2012; Vrieze A, et al. Fasting and postprandial
activity of brown fat tissue in healthy men. 53: 1407-1410
(265) J Nucl Med 2012; Lim R, et al. 18F-FDG PET/CT
metabolic tumor volume and total lesion glycolysis predict outcome
in oropharyngeal squamous cell carcinoma. 53: 1506-1513
(266) AJR 2012; Goh V, et al. Integrated 18F-FDG
PET/CT and perfusion of primary colorectal cancer: effect of inter
and intraobserver agreement on metabolic-vascular parameters. 199:
1003-1009
(267) J Nucl Med 2013; Cook GJR. Combined 18F-Fluoride
and
18F-FDG PET/CT scanning for evaluation of malignancy:
results of an International multicenter trial. 54: 173-175
(268) J Nulc Med 2013; Iagaru A, et al. Combined 18F-Fluoride
and 18F-FDG PET/CT scanning for evaluation of
malignancy: results of an International multicenter trial. 54:
176-183
(269) J Nucl Med 2013; Okamoto S, et al High repreoducibility of
tumor hypoxia evaluated by 18F-fluoromisonodazole PET
for head and neck cancer. 54: 201-207
(270) J Nucl Med 2013; Metformin- an adjunct antineoplastic
therapy- divergently modulates tumor metabolism and proliferation,
interfering with early response prediction by 18F-FDG
PET imaging. 54: 252-258
(271) J Nucl Med 2013; Massollo M, et al. Metformin temporal and
localized effects on gut glucose metabolism assessed using 18F-FDG
PET in mice. 54: 259-266
(272) J Nucl Med 2013; Menda Y, Buatti JM. PET imaging during
radiotherapy of head and neck cancer. 54: 497-498
(273) J Nucl Med 2013; Muzik O, et al. 15O PET
measurtement of blood flow and oxygen consumption in
cold-activated human brown fat. 54: 523-531
(274) J Nucl Med 2013; Hoeben BAW, et al. 18F-FLT PET
during radiotherapy or chemotherapy in head and neck squamous cell
carcinoma is an ealry predictor of outcome. 54: 532-540
(275) J Nucl Med 2013; Wong KK, Piert M. Dynamic bone imaging
with 99mTc-labeled diphosphonates and 18F-NaF:
mechanisms and applications. 54: 590-599
(276) J Nucl Med 2013; Jamar F, et al.
EANM/SNMMI guideline for 18F
FDG use in inflammation and infection. 54: 647-658
(277) J Nucl med 2013; Boktor RR, et al.
Reference range for intrapatient variability in blood-pool and
liver SUV for 18F-FDG PET. 54: 677-682
(278) J Nucl Med 2013; Walker RC, et al. Measured human dosimetry
of 68Ga-DOTATATE. 54: 855-860
(279) J Nucl Med 2013; Tehrani OS, Shields AF. PET imaging of
proliferation with pyrimidines. 54: 903-912
(280) J Nucl Med 2013; Huang C, McConathy J. Radiolabeled amino
acids for oncologic imaging. 54: 1007-1010
(281) AJR 2013; Rezai P, et al. A radiologist's guide to
treatment response criteria in oncologic imaging: functional,
molecular, and disease-specific imaging biomarkers. 201: 246-256
(282) J Nucl Med 2013; Bollineni VR, et al. PET imaging of tumor
hypoxia using 18F-fluoroazomycin arabinoside in stage
III-IV non-small cell lung caner patients. 54: 1175-1180
(283) J Nucl Med 2013; Fendler WP, et al. Validation of several
SUV-based parameters derived from 18F-FDG PET for
prediction of survival after SIRT of hepatic metastases from
colorectal cancer. 54: 1202-1208
(284) Radiographics 2013; Bhatnagar P, et al. Functional imaging
for radiation planning, response assessment, and adaptive therapy
in head and neck cancer. 33: 1909-1929
(285) J NUcl Med 2013; Noh TS, et al. Relation of carotid artery
18F FDG uptake to c-reactive
protein and Framingham risk score in a large cohort of
asymptomatic adults. 54: 2070-2076
(286) J Nucl Med 2014; Keidar Z, et al. 18F FDG uptake in noninfected
prosthetic vascular grafts: incidence, patterns, and changes
over time. 55: 392-395
(287) J Nucl Med 2014; Orlhac F, et al. Tumor
texture analysis in 18F
FDG PET: relationships between texture parameters, histogram
indices, standardized uptake values, metabolic volumes, and
total lesion glycolysis. 55: 414-422
(288) J Nucl Med 2014; Segall GM. PET/CT with
sodium 18F-fluoride for
management of patients with prostate cancer. 55: 531-533
(289) J Nucl Med 2014; Mick CG, et al.
Molecular imaging in oncology: 18F-sodium
fluoride PET imaging of osseous metastatic disease. 203: 263-271
(290) J Nucl Med 2014; Heiss WD. Clinical
impact of amino acid PET in gliomas. 55: 1219-1220
(291) J Nucl Med 2014; Tixier F, et al.
Visual versus quantitative assessment of intratumor 18F-FDG PET uptake heterogeneity:
prognostic value in non-small cell lung cancer. 55: 1235-1241
(292) AJR 2014; Keramida G, et al.
Accumulation of 18F FDG
in the liver in hepatic steatosis. 203: 643-648
(293) J Nucl Med 2014; Tahari AK, et al.
Optimum lean body formulation for correction of standardized
uptake value in PET imaging. 55: 1481-1484
(294) Radiographics 2014; Bastawrous S, et
al. Nwere PET application with an old tracer: role of 18F-NaF skeletal PET/CT in oncologic
practice. 34: 1295-1316
(295) Radiology 2014; Frings V, et al.
Repeatability of metabolically active tumor volume measurements
with FDG PET/CT in advanced gastrointestinal malignancies: a
multicenter study. 273: 539-548
(296) AJR 2015; Tahari AK, et al.
Two-time-point FDG PET/CT: liver SULmean
repeatability. 204: 402-407
(297) J Nucl Med 2015; 18F-fluoride PET used for treatment
monitoring of systemic cancer therapy: results from the National
Oncologic PET registry. 56: 222-228
(298) J Nucl Cardiol 2015; Ahmed F, et al.
Metal artefact reduction algorithms prevent false positive
results when assessing patients for caediac implantable
electronic deveice infection. 22: 219-220
(299) J
Nucl Cardiol 2015; Ahmed FZ, et al. Metal artifact reduction
algorithms prevent false positive rsults when assessing patients
for cardiac implantable electronic device infection. 22: 219-220
(300) J Nucl Med 2015; GrahamMM, et
al. Summary of the UPICT protocol for 18F-FDG PET/CT imaging in oncology
clinical trials. 56: 955-961
(301) J Nucl Med 2015; Yan J, et al. Impact
of image reconstruction setting on texture features in 18F-FDG PET. 56: 1667-1673
(302) J Nucl Med 2015; Sharma R, et al.
Multicenter reproducibility of 18F-Fluciclatide PET imagign in
subjects with solid tumors. 56: 1855-1861
(303) J Nucl Med 2015; Minamimoto R, et al.
Prospective comparison of 99mTc-MDP scintigraphy,
combined 18F-NaF
and 18F-FDG
PET/CT, and whole-body MRI in patients with breast and prostate
cancer. 56: 1862-1868
(304) J Nucl Med 2016; Grkovski M, et al.
Feasibility of 18F-fluoromisonidazole
kinetic modeling in head and neck cancer using shortened
acquisition times. 57: 334-341
(305) J Nucl Med 2016; Bahler L, et al.
Differences in sympathetic nervous stimulation of brown adipose
tissue between young and old, and the lean and obese. 57:
372-377
(306) Radiographics 2016; Ziai P, et al. Role
of optimal quantification of FDG PET imaging in the clinical
practice of radiology. 36: 481-496
(307) J Nucl Med 2016; Iqbal R, et al.
Multiparametric analysis of the relationship between tumor
hypoxia and perfusion with
18F-fluoroazomycin arabinoside and
15O-H2O PET. 57: 530-535
(308) J Nucl Med 2016; Burger IA, et al. 18F-FDG PET/CT of non-small cell lung
carcinoma under neoadjuvant chemotherapy: background-based
adaptive-volume metrics outperform TLG and MTV in predicting
histopathologic response. 57: 849-854
(309) J Nucl Med
2016; Apolo AB, et al. Prospective study evaluating Na18F PET/CT in predicting clinical
outcomes and survival in advanced prostate cancer. 57: 886-892
(310) Radiology 2016; O JH, et al. Practical
PERCIST: a simplified guide to PET response criteria in solid
tumors 1.0. 280: 576-584
(311) J Nucl Med 2016; Takx RAP, et al.
Supraclavicular brown adipose tissue 18F-FDG
uptake and cardiovascular disease. 57: 1221-1225
(312) AJR 2016; Kendi ATK, et al. Is there a role for PET/CT
parameters to characterize benign, malignant, and metastatic
parotid tumors? 207: 635-640
(313) J Nucl Med 2016; Iagaru AH, et al. Bone-targeted imaging
and radionuclide therapy in prostate cancer. 57: 19S-24S
(314) J Nucl Med 2016; Jadvar H. PET of glucose metabolism and
cellular proliferation. 57: 25S-29S
(315) AJR 2016; Rydzak C, et al. Fat-containing hypermetabolic
masses on FDG PET/CT: a spectrum of benign and malignant
conditions. 207: 1095-1104
(316) AJR 2017; Paschali AN, et al. One coin, no need to flip:
shared PET targets in cancer and coronary artery disease. 208:
434-445
(317) J Nucl Med 2017; Cottereau AS, et al. Baseline total
metabolic tumor volume measured with fixed or different adaptive
thresholding methods equally predicts outcome in peripheral T cell
lymphoma. 58: 276-281
(318) Radiographics 2017; Lakhani A, et al. FDG PET/CT pitfalls
in gynecoogic and genitourinary oncologic imaging. 37: 577-594
(319) J Nucl Med 2017; Lodge MA. Repeatability of SUV in
oncologic 18F-FDG-PET. 58: 523-532
(320) J Nucl Med 2017; Schwartz J, et al. Pharamacokinetic
analysis of dynamic 18F-fluoromisonidazole PET data in
non-small cell lung cancer. 58: 911-919
(321) J Nucl Cardiol 2017; Moon SH,
et al. Hepatic FDG uptake is associated with future cardiovascular
events in asymptomatic individuals with non-alcoholic fatty liver
disease. 24: 892-899
(322) J Nucl Med 2017; Ulaner GA, et al. Prospective clinical
trial of 18F-fluciclovine PET/CT
for determining the response to neoadjuvant therapy in invasive
ductal and invasive lobular breast cancers. 58: 1037-1042
(323) J Nucl Med 2017; Gerngrob C, et al.
Active brown fat during 18F-FDG-PET/CT
imaging defines a patient group with characteristic traits and an
increased probability of brown fat redection. 58: 1104-1110
(324) Radiographics 2017; Wallitt KL, et al. Clinical PET imaging
in prostate cancer. 37: 1512-1536
(325) J Nucl Med 2017; Lofgren J, et al. A prospective study
comparing 99mTc-hydroxyethylene diphosphonate planar
bone scintigraphy and whole body SPECT/CT with 18F-fluoride
PET/CT and 18F-fluoride PET/MRI for the diagnosing
bone metastases. 58: 1778-1785
(326) AJR 2018; Fan SKY, et al. Impact of interventional oncology
therapies on tumor microenvironment and strategies to enhance
their efficacy. 210: 648-656
(327) J Nucl Med 2018; Pattison DA, et al.
18F-FDG-avid thyroid incidentalomas: the
importance of contextual interpretation. 59: 749-755
(328) J Nucl Med 2018; Bailey DL, et al. Accuracy of dose
calibrators for 68Ga PET imaging: unexpected findings in a
mutlicenter clinical pretrial assessment. 59: 636-638
(329) Radiology 2018; Viglianti BL, et al. Effects of tumor
burden on reference tissue standardized uptake for PET imaging:
modification of PERCIST criteria. 287: 993-1002
(330) Radiology 2018; Meformin discontinuation prior to FDG
PET/CT: a randomized controlled study to compare 24- and 48-hour
bowel activity. 289: 418-425
(331) J Nucl Med 2019; Bruycker SD, et al. 18F-flortanidazole
hypoxia PET holds promise as a prognostic and predictive imaging
biomarker in a lung cancer xenograft model treated with metformin
and radiotherapy. 60: 34-40
(332) J Nucl Med 2019; Butof R, et al. Prognostic value of
standard uptake ratio in patients with trimodality treatment of
locally advanced esophageal carcinoma. 60: 192-198
(333) AJR 2019; Araki T, et al. Focal liver uptake on FDG PET/CT
without CT correlate: utility of MRI in the evaluation of patients
with known malignancy. 213: 175-181
(334) AJR 2019; Ulaner GA. PET/CT for patients with breast
cancer: where is the clinical impact? 213: 254-265
(335) Radiographics 2019; Tappouni RR, et al. ACR TI-RADS:
pitfalls, solutions, and future directions. 39: 2040-2052
(336) J Nucl Med 2020; Cook GJR, Goh V. Molecular imaging of bone
metastases and their response to therapy. 61: 799-806
(337) J Nucl Med 2020; Iravani A, Hicks RJ. Imaging the cancer
immune environment and its response to pharnacologic intervention,
part 1: the role of 18F-FDG PET/CT. 61: 943-950
(338) AJR 2021; Calais J, Mona CE. Will FAPI PET/CT replace FDG
PET/CT in the next decade? Point- an important diagnostic,
phenotypic, and biomarker role. 216: 305-306
(339) AJR 2021; Moradi F, Iagaru A. Will FAPI PET/CT replace FDG
PET/CT in the next decade? Counterpoint- no, not so fast! 216:
307-308
(340) J Nucl Med 2021; Altmann A, et al. The latest developments
in imaging fibroblast activation protein. 62: 160-167
(341) J Nucl Med 2021; Hicks RJ, et al. FAPI PET/CT: will it end the hegemony of 18FDG in oncology? 62: 296-302