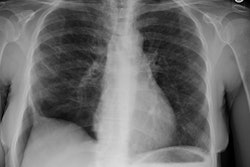
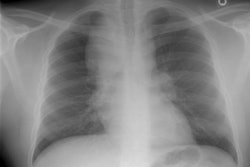
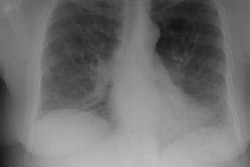
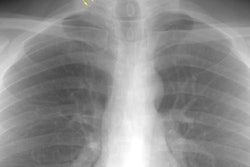
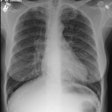
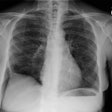
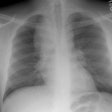
Eosinophilic Lung Disease: (Pulmonary Infiltrates with Eosinophilia)
View cases of pulmonary infiltrates with eosinophilia
The diagnosis of eosinophilic lung disease can be made if any of the following findings are present: (a) pulmonary infiltrates with peripheral eosinophilia (defined as an absolute blood eosinophil count exceeding 0.5 x 109/L), (b) tissue eosinophilia confirmed at lung biopsy, or increased eosinophils in BAL fluid of greater than 10% (normal less than 1%) [10,15].
There are 3 forms of eosinophilic lung disease:
1- Idiopathic eosinophilic lung disease:
a. Loffler's syndrome/Simple pulmonary eosinophilia
b. Acute eosinophilic pneumonia
2- Eosinophilic lung disease of specific etiology
3- Eosinophilic lung disease associated with angiitis/vasculitis
1- Idiopathic Eosinophilic Lung Disease:
A. Loffler's syndrome/Simple pulmonary eosinophilia:
Clinical:
In Loffler's syndrome patients develop transient pulmonary consolidations associated with peripheral eosinophilia. Patients can present with wheezing or a history of atopia, mild cough, fever, or dyspnea; although patients may have no pulmonary symptoms [10]. Serum analysis will commonly demonstrate a leukocytosis and an eosinophilia of at least 10% is required for the diagnosis. The prognosis is excellent and treatment is not necessary as the condition resolves within 1 month [14,15]. Although no cause can be found in up to one-third of patients, most are eventually discovered to have a drug reaction or parasitic infection [7].
CXR reveals a single (or multiple) area (s) of ill-defined, non-segmental consolidation predominantly in the lung periphery ("reverse pulmonary edema appearance") that are usually transient/migratory and resolve within one month [10]. Pleural effusion and adenopathy are not associated with this condition [14]. Less commonly, the disorder can appear as a single or multiple pulmonary nodules. On HRCT, areas of ground-glass attenuation can be seen about the areas of consolidation mainly involving the peripheral regions of the mid and upper lung zones [7,10]. Ill-defined airspace nodules with surrounding ground glass can also be seen [10]. Differential considerations include pulmonary hemorrhage (vasculitis), COP, and recurrent aspiration [10].
B. Acute Eosinophilic Pneumonia:
View cases
Clinical:
Idiopathic acute eosinophilic pneumonia is characterized
clinically by an acute febrile illness lasting one to five days.
Affected patients have myalgias, pleuritic chest pain, and the
rapid onset of hypoxemic respiratory failure often requiring
mechanical ventilation [14]. Symptoms peak within the first 5 days
[14]. However, less severe forms of the disorder likely exist
[12]. The average age for patients is 30 years [15]. The disorder
may idiopathic or represent a secondary hypersensitivity reaction
to a specific agent (fungal infection, vaccination,
environmental/dust exposure, or drug- minocycline, fludarabone,
progesterone) [13,15]. Some authors have suggested an association
with smoking- especially individuals that have recently started
smoking and those that have increased their daily smoking [12,14].
AEP typically develops within one month of initiation of smoking
in these cases [12].
The disease can be confirmed when the following clinical criteria
are met: duration of acute febrile illness of fewer than 5 days,
progression to hypoxemic respiratory failure, abnormal findings at
chest imaging, bronchoalveolar lavage eosinophils exceeding 25%,
prompt response to steroid therapy, and absence of underlying
infection [16].
Histologically, there is edema and eosinophils within the
alveolar space, and to a lesser degree within the interstitial
space. Lung injury results from release of eosinophilic granules.
Bronchoalveolar lavage will demonstrate an abundant eosinophilia
(over 25%) [14,15]. Peripheral blood eosinophilia may be absent
initially, but is elevated during the subsequent clinical course
[7,14]. Patients have a rapid (within 3 days) and complete
response to treatment with corticosteroids [13]. Relapse following
cessation of steroid therapy is not common. [1,2] The radiographic
abnormalities typically resolve without fibrosis [14].
X-ray:
CXR: On CXR there is initially subtle bilateral interstitial abnormalities (reticular densities) which progress rapidly (over 6 to 48 hours) to a diffuse interstitial and airspace disease (similar to pulmonary edema or ARDS in appearance). Pleural effusions are also commonly found in up to two-thirds of cases.
Computed tomography: CT scanning frequently demonstrates the presence of bilateral areas of ground-glass density accompanied by interlobular septal thickening. Areas of patchy consolidation can also be identified. Pleural effusions are common [15]. The findings may difficult to distinguish from adult respiratory distress syndrome, overhydration pulmonary edema, or an atypical bacterial or viral pneumonia. [1,2]
C- Chronic Eosinophilic Pneumonia:
View cases
Clinical:
Affected patients typically present between the ages of 30 - 50
years and women are affected more than men (2:1) [10,15]. Most
patients complain of a few month history (average about 4-8 months
[10,14]) of mild symptoms, such as adult onset asthma (about
30-50% of cases), allergic rhinitis, and constitutional symptoms
of low grade fever, weight loss, or malaise; however, dyspnea and
a more acutely symptomatic presentation can occur.
No specific etiology has been described, but the disorder may
also represent a hypersensitivity reaction (to drugs such as
nonsteroidal antiinflammatory agents, salicylates, minocycline,
cortrimoxazole, fludarabone, or progesterone, or to a fungal or
parasitic infection) [13]. Peripheral eosinophilia is present in
up to 85-90% of affected patients and it is usually mild or
moderate (more than 1000 uL) [10,14,15]. However, peripheral
eosinophilia may be absent at the time of presentation in up to
one-third of patients. Sputum eosinophilia is present in 50% of
patients [15]. Increased IgE levels are seen in 2/3d's of patients
and the ESR is usually elevated [10]. Eosinophils are also found
in the lung interstitium- bronchoalveolar lavage (BAL) fluid will
often contain 20 to 40% eosinophils which normally constitute less
than 1% of cells found on BAL.
There is a dramatic response to steroid therapy, but prolonged therapy (6 months) is often required and the relapse rate is high (30-60% of patients after cessation of steroids [15]). [3,4,5,6]
X-ray:
CXR: The classic CXR findings are similar to Loffler's syndrome with patchy, peripheral air space consolidations with a predilection for the upper lobes (a photographic negative of a pulmonary edema type pattern is seen in 25-34% of cases [14]). Unlike Loffler's, however, the infiltrates do not change rapidly and are slow to clear. The peripheral distribution of the infiltrates may not be evident by plain film in more than 50% of cases, in which case, patchy non-segmental areas of consolidation will be identified. Nodules are present in 20% of cases, while pleural effusions are found in under 10% of cases [7,10].
Computed tomography: A discussion of the CT findings in patients with chronic eosinophilic pneumonia is provided below. It should be noted, that findings have been described in only a small number of patients (see references).
On HRCT, patchy bilateral, peripheral, non-segmental regions of consolidation that often have sharp demarcation from normal lung and mainly involve the upper lobes [10]. Non-peripheral regions of consolidation may also be seen. Some of the opacities may appear streaky or band-like. Cavitation within areas of peripheral infiltrate has been described. Interlobular interstitial thickening and intralobular reticular opacities have been described in association with the areas of consolidation. Areas of ground glass opacification are also commonly seen. Mediastinal adenopathy has been reported in half the cases in one study, but the number of patients studied was small. The appearance may be indistinguishable from BOOP. Following institution of steroid treatment, the consolidations can begin to clear in as soon as two weeks- typically from the periphery. [3,4,5,6]
D- Idiopathic Hypereosinophilic Syndrome:
Clinical:
Idiopathic hypereosinophilic syndrome is a rare, fatal,
multiorgan system disorder characterized by blood eosinophilia
(greater than 1,500/ml) for over 6 months; the absence of
parasitic, allergic, or other known causes of eosinophilia; and
evidence of organ involvement and mutliorgan damage/dysfunction
[10,14]. Patients are usually between the ages of 30-50 years and
males are affected seven times more frequently than women [10].
The condition is a multisystem disorder which can involve nearly
every organ system, but the heart and CNS (encephalopathy and
peripheral neuropathy) are generally involved and are a major
source of morbidity and mortality [10,14]. Severe cardiac
dysfunction occurs in 60-75% of cases, often in the form of a
restrictive cardiomyopathy with endocardial fibrosis [15].
Pulmonary involvement is found in 40% of cases [10]. The BAL fluid
eosinophilia can be as high as 70% [10]. Skin involvement causes
urticaria and angioedema) [14].
Patients have historically been treated with steroids, but the
prognosis remained poor with a mortality rate of approximately 75%
in two years [15]. The fusion protein FIP1L1-PGRFRα is elevated in
up to 50% of affected patients and these patients typically
respond to treatment with the protein kinase inhibitor imantinib
[10- Invited commentary]. Use of cytotoxic drugs and monoclonal
antibodies has resulted in improved prognosis with a mortality
rate of approximately 4% within three years of diagnosis [15].
Findings on the chest radiograph are varied and non-specific
including patchy areas of reticular densities, poorly defined
nodules, or consolidation. Pulmonary edema can be seen secondary
to cardiac dysfunction [10]. Pleural effusions can be found in up
to 50% of cases. Areas of ground-glass attenuation and
consolidation in a patchy and peripheral may be identified at CT
(similar to chronic eosinophilic pneumonia [14]). [7]
2- Eosinophilic Lung Disease of Specific Etiology:
The clinical and radiographic findings are similar to that of idiopathic eosinophilic lung disease, however, an etiologic agent can be identified. Etiologies include:
- Drugs: Also referred to as "drug induced eosinophilic lung disease." Drug induced eosinophilic pneumonias can be acute or insidious in onset [9]. Acute injury is usually mild and self-limited [9]. The presentation of chronic disease can vary from minimal symptoms to severe illness with malaise, dyspnea, and fever [9]. Agents implicated include amiodarone [9], nitrofurantoin [9], methotrexate [9], sulfonamides[9], aspirin and nonsteroidal anti-inflammatory agents [9], beta-blockers [9], penicillin, L-tryptophan [10], and aerosolized pentamidine. Peripheral eosinophilia (seen in about 40% of patients [up to 86%]) [9] and elevated IgE levels are common. Lung injury is unrelated to the cumulative dose [9]. Prognosis is generally good if the offending agent is discontinued, but in some cases steroid therapy may be required [8,9]. Peripheral opacities are common on CXR. On HRCT the most common finding is air-space consolidation and areas of ground glass attenuation [9]. These findings tend to involve the lung periphery [9]. Other findings include small centrilobular nodules and interlobular septal thickening [9].
- Parasites: Many parasites can cause pulmonary opacities with blood or tissue eosinophilia [10]. Two mechanisms of pulmonary eosinophilic infiltration in parasitic infections have been postulated- direct invasion of the lung parenchyma (Ascaris, Schistosoma, Paragonimus westermani, Ancylostoma duodenale, and Filaria species such as Wuchereria bancrofti and Brugia nakayi) and allergic reaction (Entamoeba hsitolytica, Toxocara canis, and Clonorchis sinensis) [10]. See individual infections under- Ascariasis, Schistosomiasis, Strongyloides, or Viseral larva migrans
- Fungi: Allergic bronchopulmonary aspergillosis
- Pulmonary eosinophilia with asthma.
- Cocaine inhalation [11]
3- Eosinophilic Lung Disease associated with Angiitis/ Granulomatosis:
Due to Churg-Strauss, Bronchocentric granulomatosis, Wegener's granulomatosis, Polyarteritis nodosa, or Sjogren's syndrome.
REFERENCES:
(1) AJR 1996; Nov 167 (5): 1195-1199
(2) Radiology 1997; Jun 203 (3): 715-719
(3) J Comput Assist Tomogr 1994, 18 (5): 737-744
(5) Medicine 1988; 67: 154-162
(6) Chest 1994; May 105 (5): 1462-66
(7) J Comput Assist Tomogr 1997; 21 (6): 920-930
(8) Society of Thoracic Radiology Annual Meeting 2000 Course Syllabus; Erasmus JJ. Pulmonary drug toxicity: Pathogenesis and radiologic manifestations. 65-68
(9) AJR 2006; Souza CA, et al. Drug-induced eosinophilic pneumonitis: high-resolution CT findings in 14 patients. 186: 368-373
(10) Radiographics 2007; Jeong YJ, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. 27: 617-639
(11) Radiographics 2007; Restrepo CS, et al. Pulmonary complications from cocaine and cocaine-based substances: imaging manifestations. 27: 941-956
(12) Chest 2008; Uchiyama H, et al. Alterations in smoking habits
are asscociated with acute eosinophilic pneumonia. 133: 1174-1180
(13) AJR 2013; Nemec SF, et al. Noninfectious inflammatory lung
disease: imaging considerations and clues to differential
diagnosis. 201: 278-294
(14) Radiographics 2016; Katre RS, et al. Cardiopulmonary and
gastrointestinal manifestations of eosinophil-associated diseases
and idiopathic hypereosinophilic syndromes: multimodality imaging
approach. 36: 433-451
(15) AJR 2017; Bernheim A, McLoud T. A review of clinical and
imaging findings in eosinophilic lung diseases. 208: 1002-1010
(16) Radiology 2020; Kligerman S, et al. Radiologic, pathologic,
clinical, and physiologic findings of electronic cigarette or
vaping product use- associated lung injury (EVALI): evolving
knowledge and remaining questions. 294: 491-505