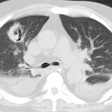
Aspergillosis:
Exposure to Aspergillus is universal, however, the normal host defenses protect most people from infection. Impairment of normal defenses due to immunosuppression, cavitary lung disease, or cystic fibrosis can result in infection.
View cases of Aspergillus
There are primarily 4 manifestations of disease-
1- Allergic Bronchopulmonary Aspergillosis:
Clinical:
Allergic bronchopulmonary aspergillosis (ABPA) affects patients with intact immune systems and usually begins in childhood. A. fumigatus is the most common allergen in ABPA, however, other members of the Aspergillus genus can also produce a similar disease. ABPA occurs in 1-2% of individuals with asthma and in about 10% of patients with cystic fibrosis. ABPA is both a type I (IgE) and type III (Antigen/Antibody complex) hypersensitivity reaction to Aspergillus colonizing the bronchial lumens (not the distal airways) which may mimic asthma or chronic bronchitis. Acting as a stimulant for IgE and IgG production the inflammatory reaction results in cellular and eosinophilic infiltrates [19]. Release of proteolytic enzymes produces tissue damage to the bronchial walls [19]. Excessive mucous production and abnormal ciliary function lead to mucoid impaction in the airways [19]. The disorder can be divided into 5 stages- acute, remission, exacerbation, corticosteroid dependent, and fibrotic [22].
Approximately 96% of patients with ABPA will have symptoms of asthma (wheezing) (the prevalence of ABPA is repported to ne 2-32% in patients with asthma [23]). Patients may also have flu-like symptoms with fever, malaise, and fatigue. Patients may cough up thick mucous plugs in which hyphal fragments can be demonstrated [19]. Between exacerbations the flu-like symptoms will resolve, but the asthma may continue. If untreated, the condition will progress to lung fibrosis with chronic dyspnea. Other findings include serum eosinophilia (typically between 8-40% [normal is 1-4%]; eosinophilia greater than 40% suggests another diagnosis), sputum eosinophilia, elevated serum IgG, positive skin test for Aspergillus, and recurrent pulmonary opacities. About 50% of affected patients produce brownish sputum which microscopically reveal Aspergillus hyphae. Sputum cultures can also be positive for A. fumigatus, but hyphae should be identified to confirm the diagnosis as A. fumigatus is an ubiquitous organism and may be a contaminant.
Both primary and secondary diagnostic criteria exist for ABPA. Primary criteria include: (ARSEPICS) A- asthma; R- roentgenographic pulmonary infiltrates; S- Skin test positive for A. fumigatus (may be masked by steroid therapy [11]); E- eosinophilia; P- precipitating antibodies to A. fumigatus (most specific test for ABPA, but may be masked by steroid therapy [11]); I- IgE in serum elevated (may be masked by steroid therapy [11]); C- central bronchiectasis; and S- serum specific IgE and IgG A. fumigatus levels elevated. If 6 of these 8 criteria are met, the diagnosis of ABPA can be made with reasonable certainty. Central bronchiectasis can confirm the diagnosis, but it is a late manifestation of the disorder. Secondary criteria include A. fumigatus in the sputum, brownish sputum plugs, and Arthus reactivity to Aspergillus antigen.
Treatment is oral steroids which is effective at minimizing damage to the airways and often hastens the resolution of pulmonary infiltrates. [8]
Other fungi that may cause a similar syndrome in asthmatics include Fusarium vasinfectum, Schizophyllum commune, Candida species, and Pseudallescheria boydii [11].
X-ray:
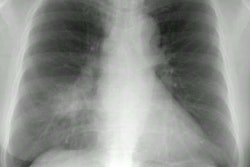
On plain film the most common manifestation of ABPA is transient parenchymal opacities ("fleeting shadows") which are found in 80-90% of cases, but the CXR can be normal is as many as 50% of cases during the acute phase [23]. These opacities are most likely the result of airway obstruction with atelectasis. Bronchiectatic or normal bronchi are commonly impacted with mucus, hyphae, and debris. The impaction may extend from the proximal bronchi into the second-, third-, or fourth-order bronchi and this produce a "gloved-finger" or tubular "branching" radiodensities. In about 30% of cases, the inspissated mucus in the bronchus often has a CT density greater than that of soft tissue- similar to the high-density material seen in the paranasal sinuses of patients with fungal sinusitis (the high-attenuation mucous is attributed to the presence of calcium salts and metals (iron and manganese) or desiccated mucous [11,20,23]. Central saccular bronchiectasis with an upper lobe predominance is a characteristic end result of the disorder [20], but bronchiectasis is also commonly seen in the more distal bronchi [11] as well as within the lower lobes [15]. It is most likely the sequella of chronic inflammatory bronchial damage. Other CT findings include centrilobular nodules [20]. Aspergillomas are seen in 7% of patients with ABPA.
2- Mycetoma:
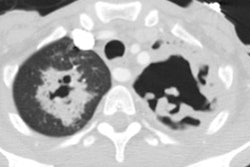
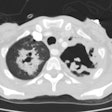
Mycetomas develop in patients with underlying cavitary lung disease (TB, sarcoid) or lung cysts due to saprophytic colonization of the cavity. Classically the cavity is thin walled and contains a mobile rounded fungus ball, but the wall may be thickened or irregular. Very rarely, Aspergillomas have been described within cavitary lung cancers [5,6,7]. Aspergillus is cultured from the sputum of affected patients in about 50% of cases, but serological tests can confirm the diagnosis. Approximately 10% of myecetomas resolve spontaneously [19]. The diagnosis carries a long-term risk of life-threatening hemorrhage of approximately 10-20%. Treatment for severe hemoptysis includes selective bronchial artery embolization or surgical resection [19].
3- Semi-invasive Aspergillosis:
Also referred to as "chronic necrotizing aspergillosis". An indolent pulmonary infection typically seen in patients with underlying non-cavitary lung disease (such as pulmonary fibrosis, emphysema, or bronchiectasis) or those who are mildly immunocompromised (low-dose corticosteroid therapy or chronic debilitating illness such as diabetes, malnutrition, or alcoholism) [17,19]. Affected patients usually complain of a chronic cough, constitutional symptoms, and hemoptysis (15% of patients) [19]. Radiographs reveal an indolent consolidation that tends to involve the upper lobes with associated pleural thickening. Upper lobe nodules or a mass-like consolidation can also be seen. It usually progresses over a period of weeks to months to cavitation in about half the cases (typically thick walled) and mycetoma formation occurs in up to 50% [14]. Treatment requires IV amphotericin B, oral itraconazole, or both [16].
4- Invasive Aspergillosis:
A) Parenchymal Invasive Aspergillosis:
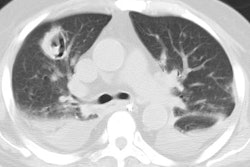
Invasive aspergillosis almost always occurs in the immunocompromised patient- most commonly granulocytopenic acute leukemic patients (absolute neutrophil count less than 500). Other conditions which predispose patients to infection include myeloablative chemotherapy, corticosteroid use, allogeneic bone marrow/stem cell transplant, organ transplant, and late-stage HIV infection [13,14,21]. Early diagnosis is essential, unfortunately sputum cultures are positive in only 10% of patients [18]. The presence of pulmonary infiltrates in a febrile, neutropoenic patient already on broad spectrum antibiotic therapy should signal the diagnosis of fungal disease. Intravenous amphotericin B is the only reliable method of therapy, and early treatment can improve survival [13]. Pathologically the disease is characterized by vascular invasion and thrombosis which leads to hemorrhagic infarction. Over time, with retraction of the infarcted center and peripheral resorption of necrotic tissue by leukocytes, a central area of devitalized tissue is formed. The "air crescent" sign results when air fills the space between the devitalized tissue and the surrounding parenchyma [18]. Mortality varies from 30 to 90% and depends on the early institution of therapy, the severity of the patients underlying disease, and the speed of granulocyte recovery [14]. Systemic dissemination occurs in 25-50% of patients [14].
On plain films there are wedge-shaped segmental or sub-segmental pulmonary infiltrates (representing areas of infarction) which mimic pneumonia. In many instances the infiltrates have a poorly defined nodular appearance. These opacities can coalesce into larger consolidations. The size of the lesion can increase within the first week of diagnosis despite adequate therapy [21]. Crescent shaped cavitation occurs within the areas of consolidation ("air crescent" sign) up to 2 weeks after the appearance of the initial radiographic abnormality [18]. The finding is due to sloughed necrotic lung surrounded by a rim of air. Cavitation is a late finding in invasive pulmonary aspergillosis indicating an immune response (neutrophil recovery) to the infection and is associated with a better prognosis than consolidation without cavitation. Patients with neutropenia do not develop cavitary lesions [18]. Cavitation can be seen in up to 50% of patients and is highly suggestive of invasive pulmonary aspergillosis, however, recognition of infection at this stage implies that treatment has been delayed [18]. Pleural effusion is uncommon and adenopathy is rare.
On HRCT, invasive aspergillus infection can appear as segmental
areas of consolidation plus adjacent ground-glass attenuation, as
nodules or nodular infiltrates with a surrounding halo of
ground-glass attenuation (CT "halo sign"). The halo represents
surrounding hemorrhagic necrosis and it can be seen in up to 88%
of patients [21], but the finding is not specific as it can also
be seen in mucormycosis, candidiasis, CMV, and herpes simplex
pneumonias. The presence of air crescents surrounding the areas of
nodular infiltration represents air between the retracted,
infarcted lung and the adjacent normal lung parenchyma are a later
finding. The crescent sign is found in about 25% of patients 16
days following diagnosis and in 45% after 32 days [21]. Wedge
shaped areas of consolidation due to infarction may also be
identified on HRCT.
A "reverse halo sign" or "atoll sign" appears as an area of
central ground-glass attenuation surrounded by a crescentric ring
of more dense air-space consolidation [24]. In an immune
compromised patient, this sign can be seen in association with
both bacterial pneumonia and fungal infection, particularly
aspergillosis and mucormycosis [24].
B) Aspergillosis in the HIV patient:
Aspergillosis is the most common pulmonary fungal pathogen in HIV patients [10], although this may be controversial as other authors state Cryptococcus is the most common (see discussion under HIV infection). In autopsy series, the overall incidence of aspergillosis infection in HIV patients is 4.5% (Range 0% to 12.5%) [12]. None-the-less, the incidence of aspergillus infection has increased likely due to prolonged survival of patients with low CD4 counts, the widespread use of drugs that may cause neutropenia (such as zidovudine and ganciclovir), and the increased use of corticosteroids [12]. Infection typically occurs late in the course of HIV infection (CD4 count less than 50 cells/uL), often in association with other risk factors such as steroid therapy, granulocytopenia (< 1,000/mm3), or IV drug use. Four unique sub-types of aspergillus infection have been described in HIV patients [2,3,10].
i) Invasive aspergillosis: Invasive aspergillosis is the most common manifestation of aspergillus infection in HIV patients. Patients commonly present with fever, cough, and dyspnea [12]. Co-morbid opportunistic infection with PCP or CMV is common [12]. BAL is often suggestive of the diagnosis in the appropriate clinical setting, but biopsy specimens are necessary for definitive diagnosis [12]. Treatment is with amphotericin B, but mortality remains high (over 80%) [12]. Thick-walled cavitary lesions (mainly in the upper lobes) are the most common radiologic manifestation of invasive pulmonary aspergillosis in HIV patients (found in up to 80% of cases) [10]. Air space consolidation, parenchymal nodules with a surrounding halo of ground-glass, and variable cavitation secondary to infarction, and pleural effusion can also be seen. The air crescent sign, a feature seen in other immune compromised patients, is infrequently seen in association with HIV invasive aspergillus infection [12]. A proposed explanation is that this sign is associated with rapid recovery from severe immunosuppression, a phenomenon that is unusual amoung HIV-infected patients [12].
ii) Necrotizing tracheobronchitis: Approximately 10% of aspergillus infections in HIV patients involve the airways. Patients present with cough, fever, wheezing, and stridor. There is ulceration and plaque-like inflammatory lesions within the airways with little or no extension into the adjacent lung parenchyma. The chest radiograph is normal in about half of the cases. Subtle irregularity/nodularity of the tracheobronchial walls may be seen on CT scan. Inflammatory exudate and mucous produce airway plugging which results in atelectatic changes.
iii) Obstructing bronchopulmonary aspergillosis: This is an unusual manifestation of infection that may be unique to HIV patients in which there is massive non-invasive intraluminal overgrowth of aspergillus [19]. Although the airways are occluded with fungi, the bronchial wall is not inflamed and the disorder represents a stage before frank tissue invasion occurs. Affected patients present with the acute onset of fever, dyspnea, and cough (with expectoration of fungal casts). Radiographically there are bilateral, predominantly lower lobe opacities due to post-obstructive atelectasis. Branching tubular shadows representing the mucoid/fingal filled bronchi can be seen.
iv) Chronic cavitary parenchymal aspergillosis: This is an unusual variant of aspergillosis involving the small airways. Necrotic debris and aspergilli fill the respiratory bronchioles and extend into alveolar air spaces, without bronchial wall invasion. Cavitary lesions appear to extend from the involved airways.
C) Invasive Aspergillosis of the Airways:
Invasive aspergillosis of the airways occurs in immune compromised hosts (neutropenic or HIV patients) [19]. Clinical manifestations include acute tracheobronchitis, bronchitis, and bronchopneumonia [19]. Only limited information is available regarding the radiologic manifestations of this form of infection which is usually recognized clinically at bronchoscopy or histologically after airway biopsy. Consolidation, nodular peribronchial consolidation, or small centrilobular nodules may be seen [4]. Occasionally, tracheal or bronchial wall thickening may be evident radiographically [19] Treatment is with IV amphotericin B, but mortality is still high (over 50% in one study). [4]
Other Manifestations of Aspergillosis:
Other less common manifestations of Aspergillus include Bronchocentric granulomatosis- an uncommon disorder characterized by granulomatous lung destruction centered around the bronchi. Affected patients are almost universally asthmatic and have blood eosinophilia. Patients complain of a persistent cough and are usually skin test positive for Aspergillus antigen. Radiographically patients may demonstrate a solitary nodule, multiple nodules, or an area of parenchymal consolidation.
REFERENCES:
(1) Semin Roentgenol 1996; Jan 31(1):52-66
(2) Radiology 1995, 196: 409-414
(4) Radiology 1994; 193: 383-388
(5) AJR 1994; Aspergilloma within cavitary lung cancer: MR imaging findings. 163: 565-567 (No abstract available)
(6) Am J Clin Path 1989; 91: 100-103
(8) Radiology 1997; A review of allergic bronchopulmonary Aspergillosis. 204: 694
(9) RadioGraphics 1997; Williams SM, et al. Cases of the day: General case of the day. 17: 1597-1600 (No abstract available)
(10) Radiol Clin North Am 1997; McGuinness G. Changing trends in the pulmonary manifestations of AIDS. 35 (5): 1029-1082
(11) Radiol Clin North Am 1998; Lynch DA. Imaging of asthma and allergic bronchopulmonary mycosis. 36 (1): 129-142
(12) Chest 1998; Mylonakis E, et al. Pulmonary aspergillosis and invasive disease in AIDS. Review of 342 cases. 114: 251-262
(13) Radiology 1998; Won HJ, et al. Invasive pulmonary aspergillosis: Prediction at thin-section CT in patients with neutropenia- a prospective study. 208: 777-782
(14) J Thorac Imaging 1999; Connolly JE, et al. Opportunistic fungal pneumonia. 14: 51-62
(15) AJR 1999; Ward S, et al. Accuracy of CT in the diagnosis of allergic bronchopulmonary aspergillosis in asthmatic patients. 173: 937-942
(16) AJR 2000; Franquet T, et al. Semiinvasive pulmonary Aspergillosis in chronic obstructive pulmonary disease: Radiologic and pathologic findings in nine patients. 174: 51-56
(17) AJR 2000; Kim SY, et al. Semiinvasive pulmonary aspergillosis: CT and pathologic findings in six patients. 174: 795-798
(18) Radiology 2001; Abramson S. The air crescent sign. 218: 230-232 (No abstract available)
(19) Radiographics 2001; Franquet T, et al. Spectrum of pulmonary Aspergillosis: Histologic, clinical, and radiologic findings. 21: 825-837
(20) Silva CI, et al. Asthma and associated conditions: high-resolution CT and pathologic findings. 183: 817-824
(21) AJR 2006; Brodoefel H, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. 187: 404-413
(22) Radiographics 2007; Jeong YJ, et al. Eosinophilic lung
diseases: a clinical, radiologic, and pathologic overview. 27:
617-639
(23) Radiographics 2016; Katre RS, et al. Cardiopulmonary and
gastrointestinal manifestations of eosinophil-associated diseases
and idiopathic hypereosinophilic syndromes: multimodality imaging
approach. 36: 433-451
(24) AJR 2019; Thomas R, et al. Significance of the reverse halo
sign in immunocompromised patients. 213: 549-554